Year: 2023 Title: ONWARDS 1 Subtitle: Weekly Icodec versus Daily Glargine U100 in Type 2 Diabetes without Previous Insulin Type of Trial: Randomized, open-label, treat-to-target phase 3a trial Objective: To investigate the efficacy and long-term safety of once-weekly insulin icodec … Read More
Endocrinology
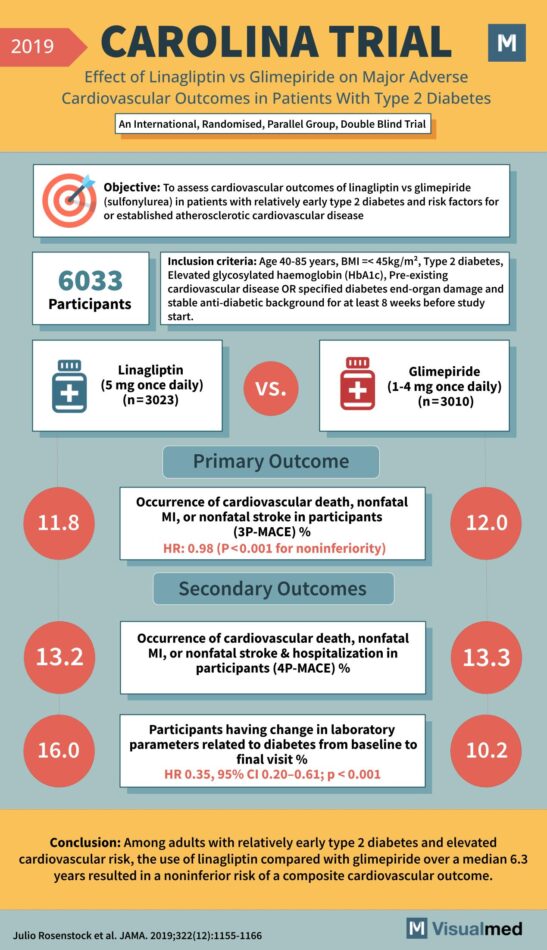
CAROLINA Trial: Linagliptin vs. Glimepiride in T2DM
Year: 2019 Title: CAROLINA TRIAL Subtitle: Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes Type of Trial: An International, Randomised, Parallel Group, Double Blind Trial Objective: To assess cardiovascular outcomes of linagliptin … Read More
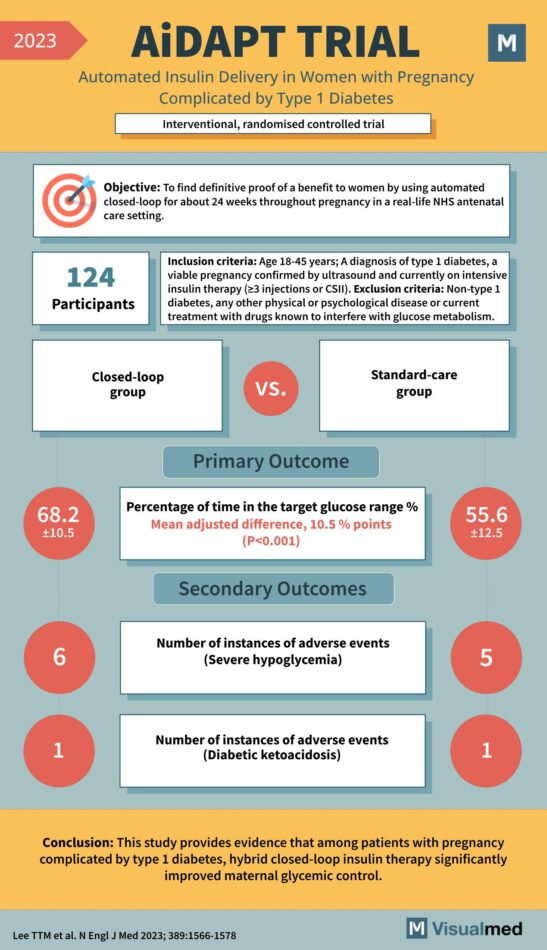
AiDAPT Trial: Automated Insulin Delivery in Pregnancy Complicated by T1DM
Year: 2023 Title: AiDAPT TRIAL Subtitle: Automated Insulin Delivery in Women with Pregnancy Complicated by Type 1 Diabetes Type of Trial: Interventional, randomized controlled trial Objective: To find definitive proof of a benefit to women by using automated closed-loop for … Read More
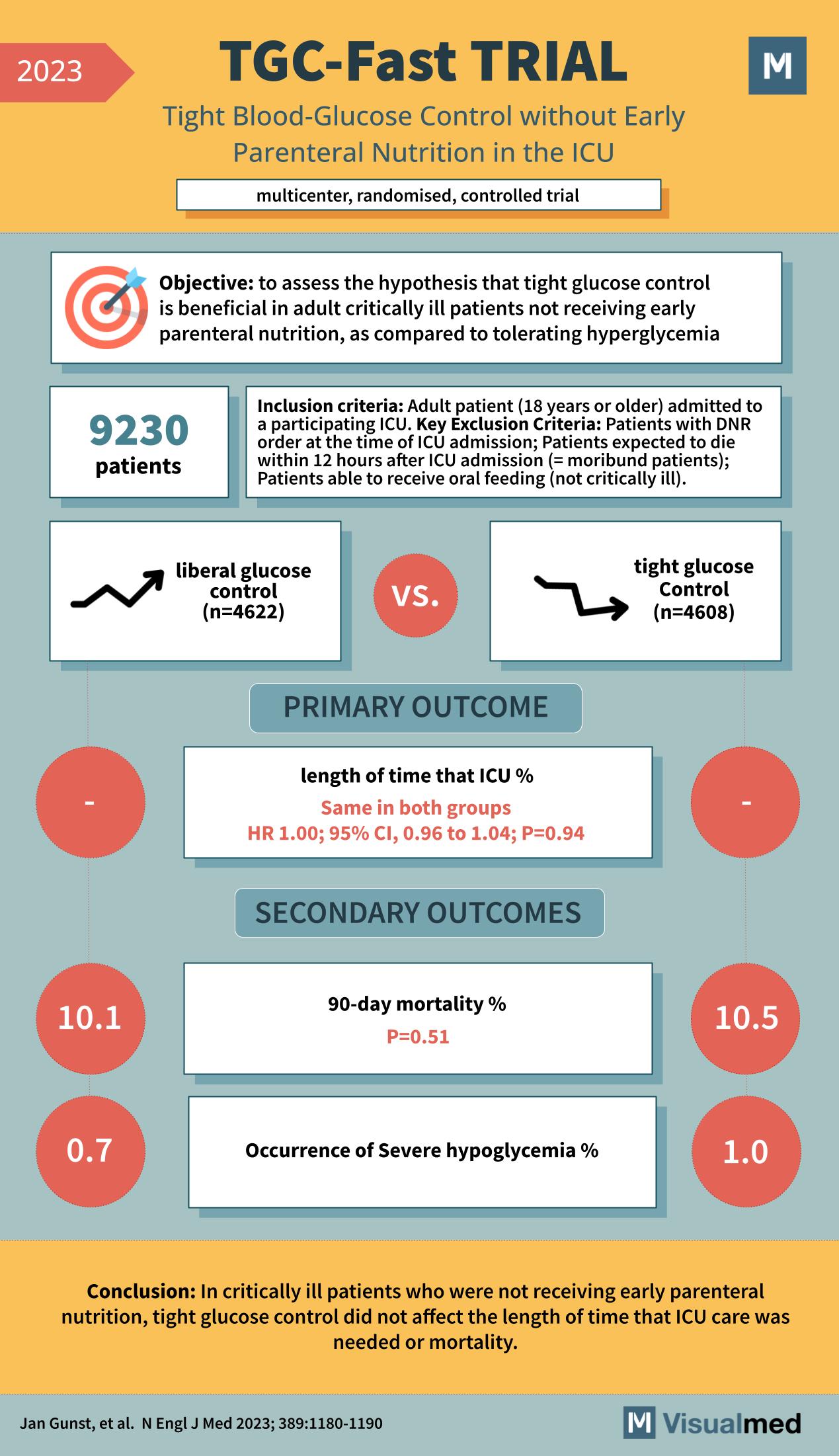
TGC-Fast Trial: Tight Glucose Control in ICU
The TGC-Fast Trial, featured in the New England Journal of Medicine in 2023, was a multicenter, randomized, controlled trial that aimed to assess the benefits of tight glucose control without early parenteral nutrition in the intensive care unit (ICU). The … Read More
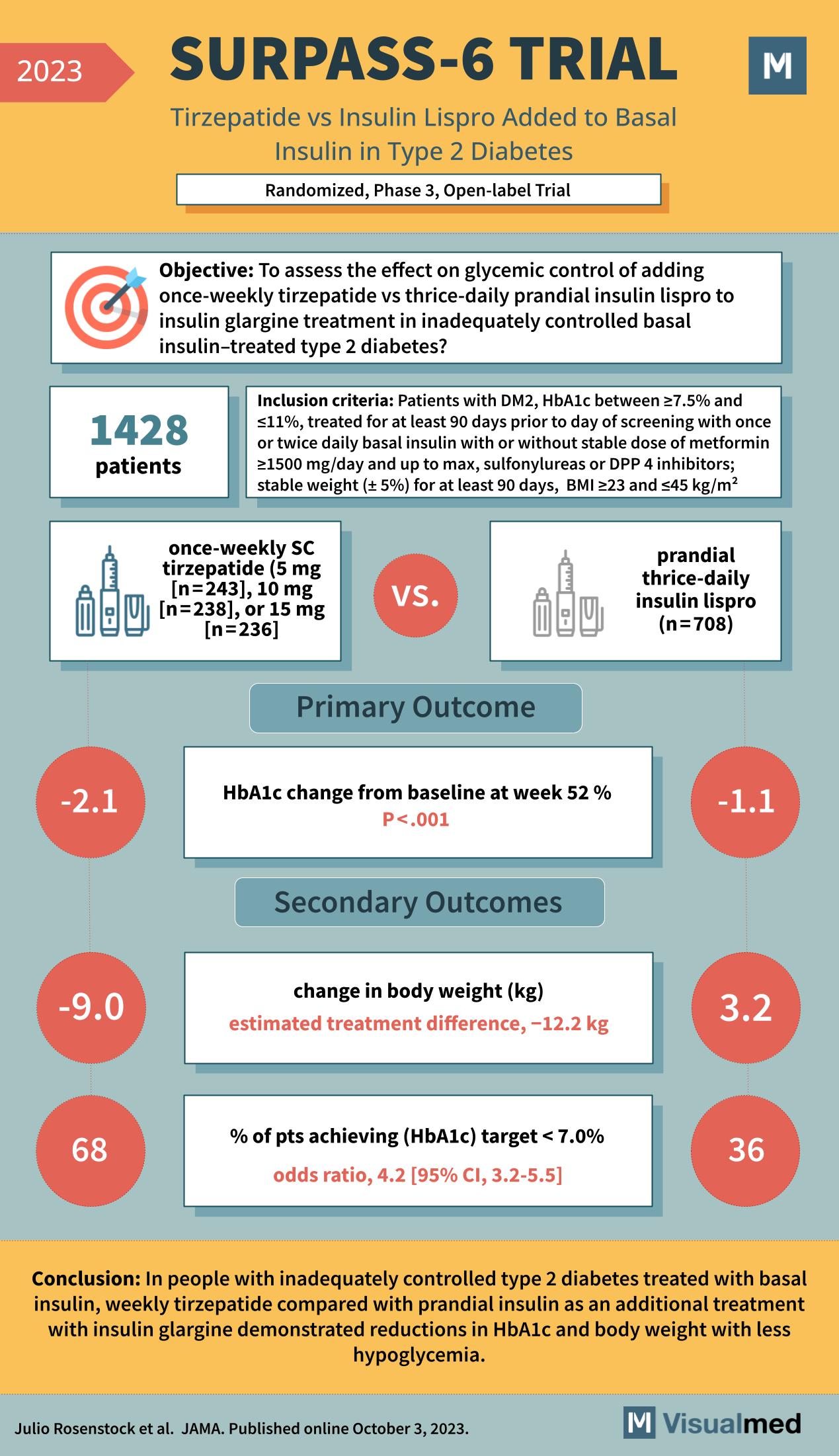
SURPASS-6 Trial: Tirzepatide in T2DM
The SURPASS-6 trial, featured in JAMA in October 2023, represents a significant advancement in the treatment of type 2 diabetes. This randomized, phase 3, open-label trial was designed to evaluate the effects of once-weekly subcutaneous (SC) tirzepatide versus thrice-daily prandial … Read More
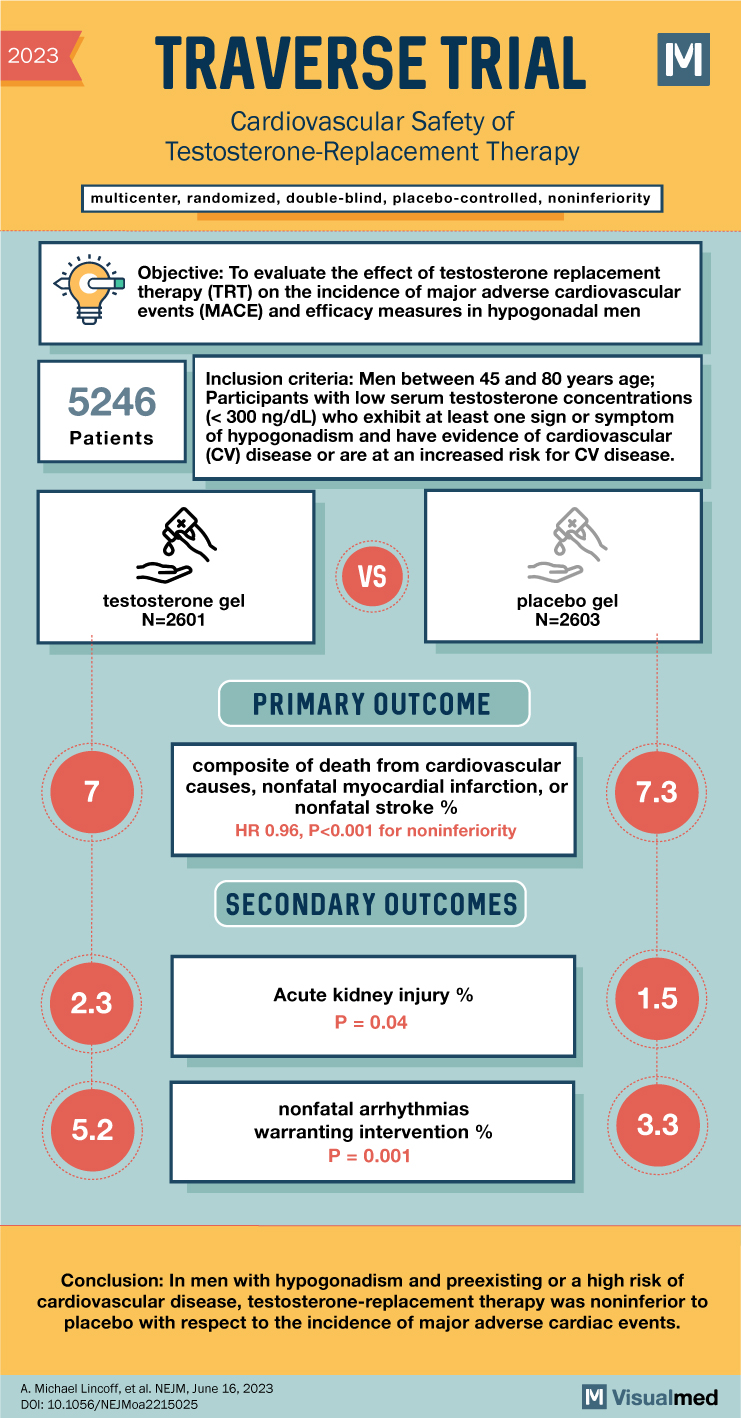
TRAVERSE Trial: CV Safety of Testosterone-Replacement Therapy
TRAVERSE Trial Summary The abstract highlights the findings of the TRAVERSE trial, which aimed to assess the cardiovascular safety of testosterone-replacement therapy in middle-aged and older men with hypogonadism. The trial sought to determine whether testosterone replacement therapy in this … Read More
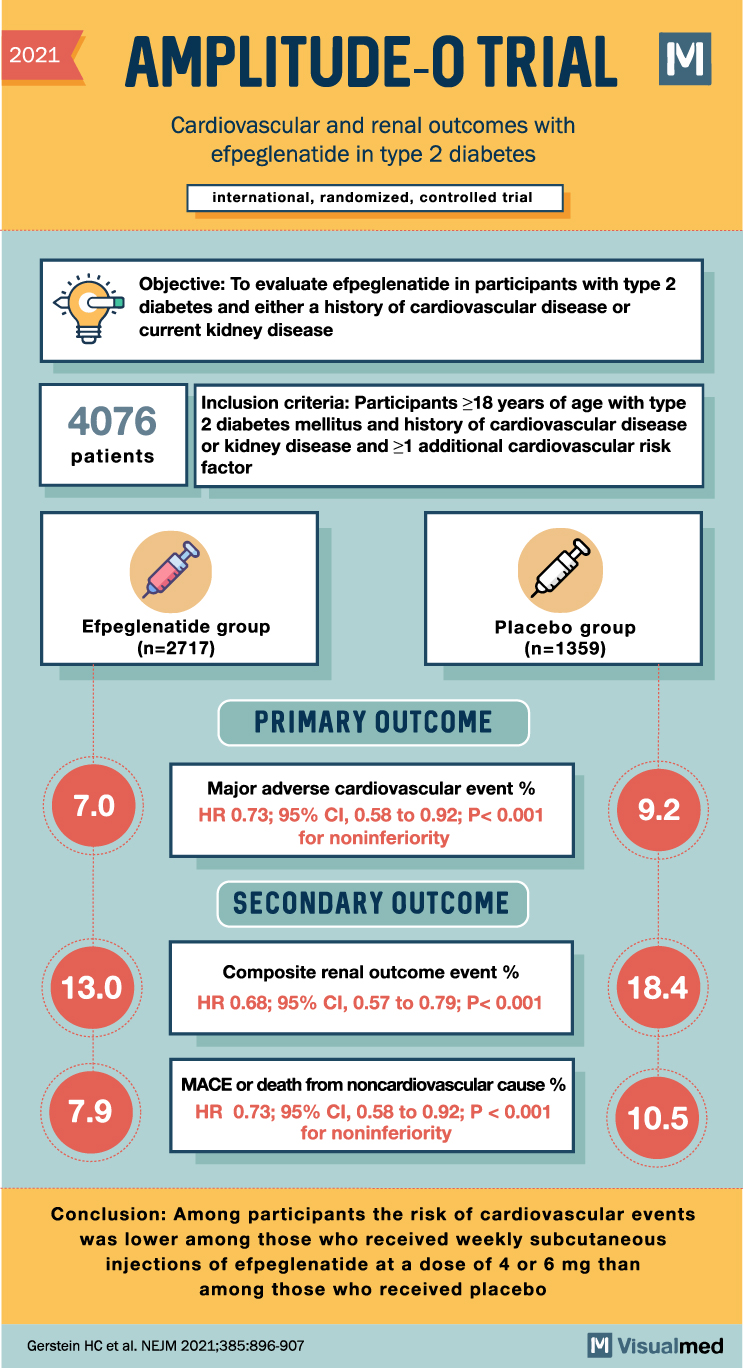
AMPLITUDE-O Trial: CV and Renal Outcomes with Efpeglenatide
2021 AMPLITUDE-O TRIAL M Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes international, randomized, controlled trial Objective: To evaluate efpeglenatide in participants with type 2 diabetes and either a history of cardiovascular disease or current kidney disease 4076 … Read More
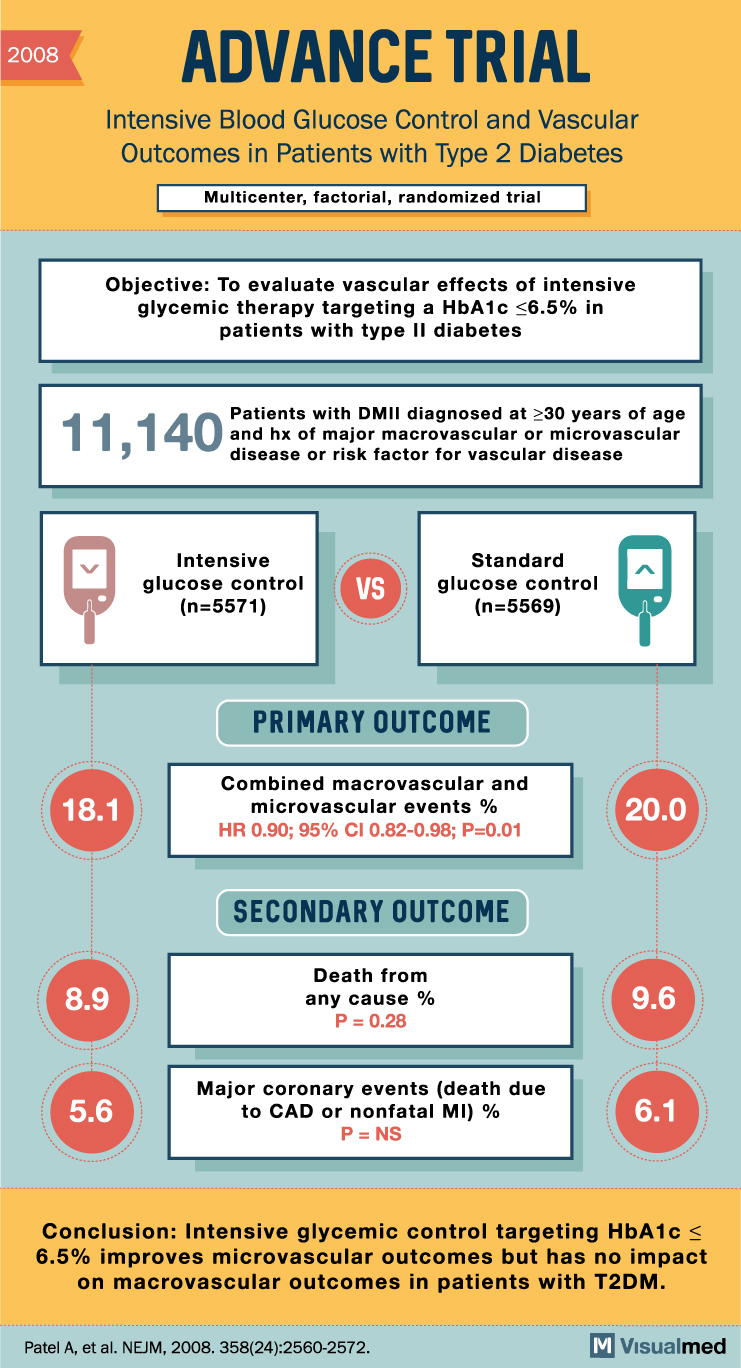
ADVANCE Trial Summary: Intensive Glucose Control in Diabetes Type 2
2008 ADVANCE TRIAL Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes Multicenter, factorial, randomized trial Objective: To evaluate vascular effects of intensive glycemic therapy targeting a HbA1c <6.5% in patients with type II diabetes 11,140 … Read More
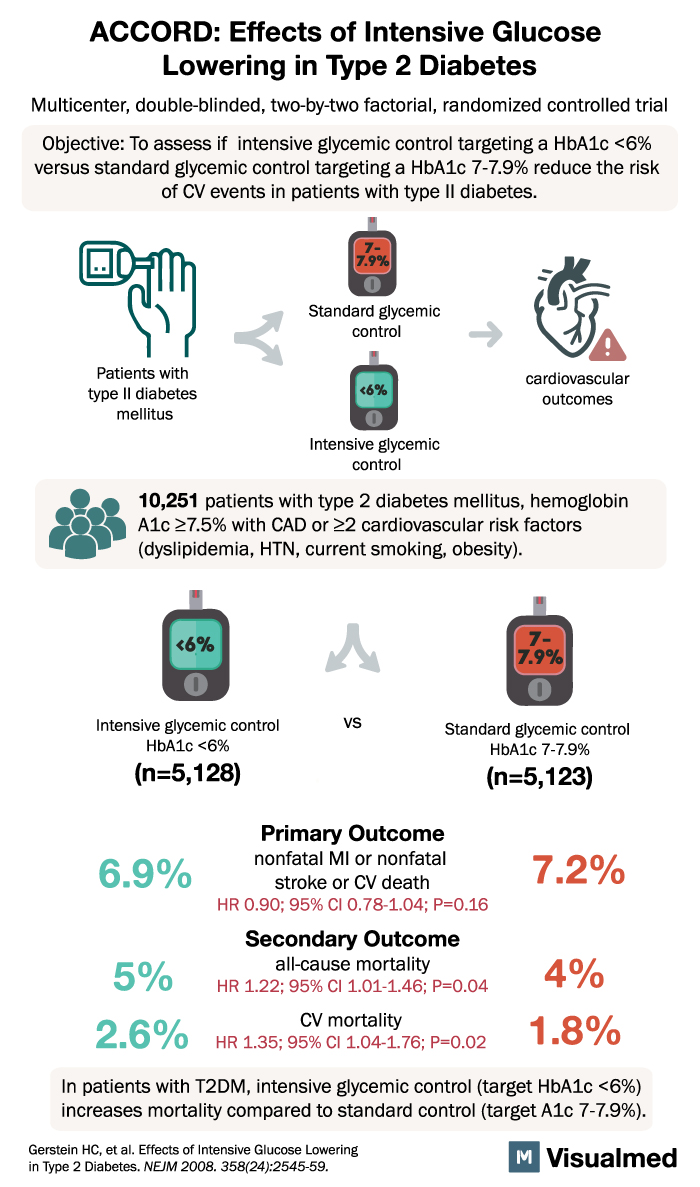
ACCORD Trial: Intensive Glucose Lowering in DM2
ACCORD: Effects of Intensive Glucose Lowering in Type 2 Diabetes Multicenter, double-blinded, two-by-two factorial, randomized controlled trial Objective: To assess if intensive glycemic control targeting a HbA1c <6% versus standard glycemic control targeting a HbA1c 7-7.9% reduce the risk of … Read More
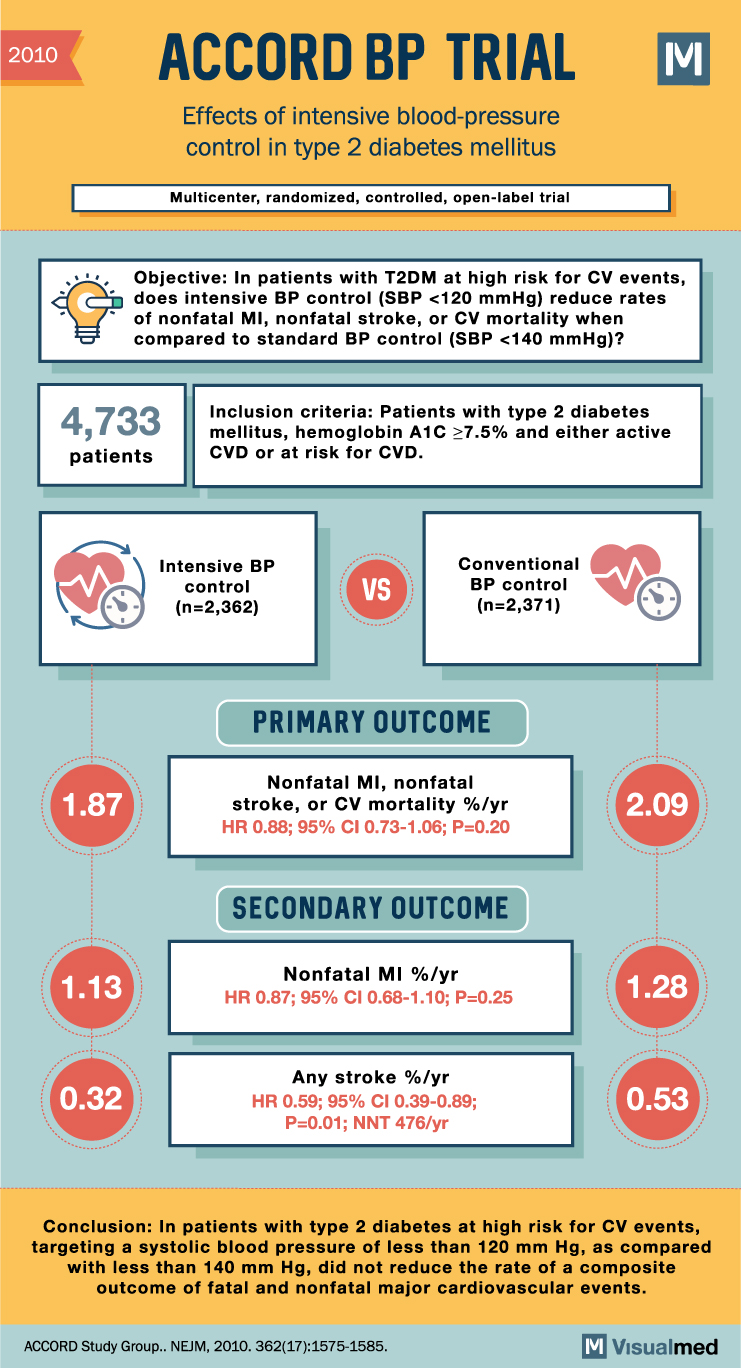
ACCORD BP Trial Summary: Intensive BP Control in Type 2 Diabetes
2010 ACCORD BP TRIAL Effects of intensive blood-pressure control in type 2 diabetes mellitus Multicenter, randomized, controlled, open-label trial Objective: In patients with T2DM at high risk for CV events, does intensive BP control (SBP <120 mmHg) reduce rates of … Read More
