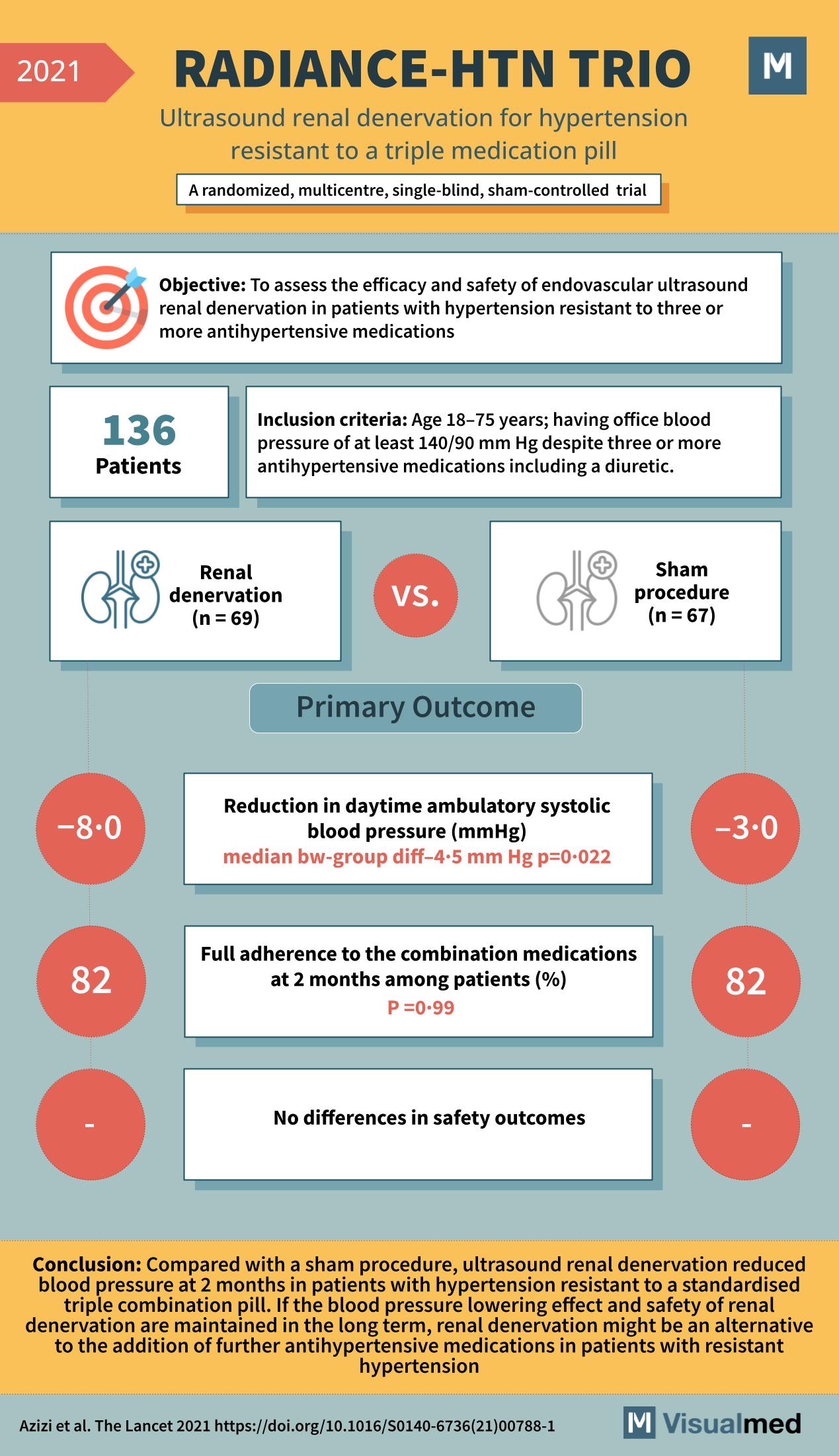
The RADIANCE-HTN TRIO trial, detailed in The Lancet in 2021, is another pivotal study in the domain of hypertension treatment, focusing on a challenging group of patients: those resistant to a standard triple combination pill. This trial delves into the … Read More
Nephrology
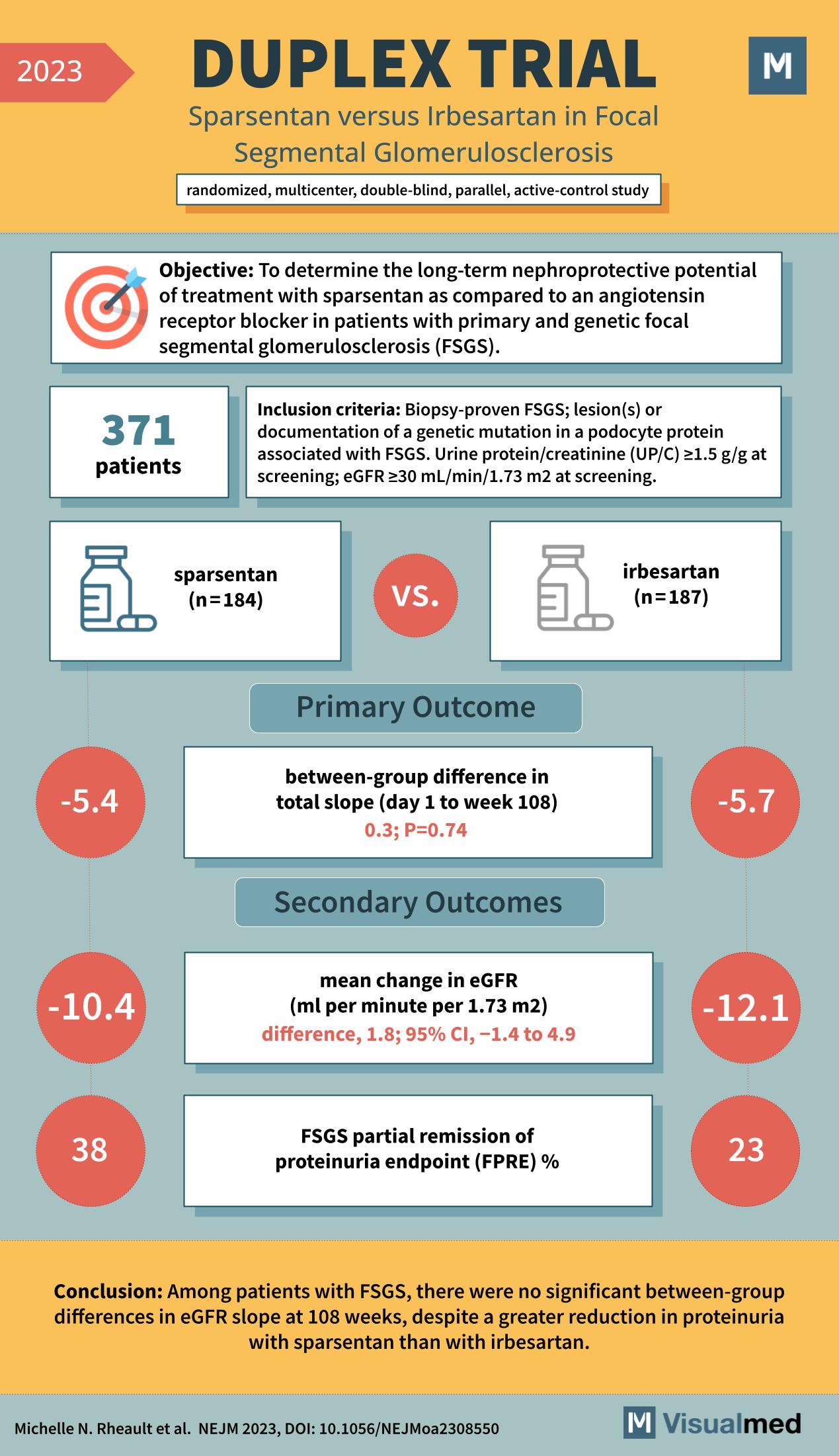
DUPLEX Trial: Sparsentan in FSGS
The DUPLEX Trial: Evaluating Kidney Protection in FSGS Patients The DUPLEX trial, a rigorous study published in the New England Journal of Medicine in 2023, has garnered significant attention in the nephrology community. The trial investigated the long-term nephroprotective potential … Read More
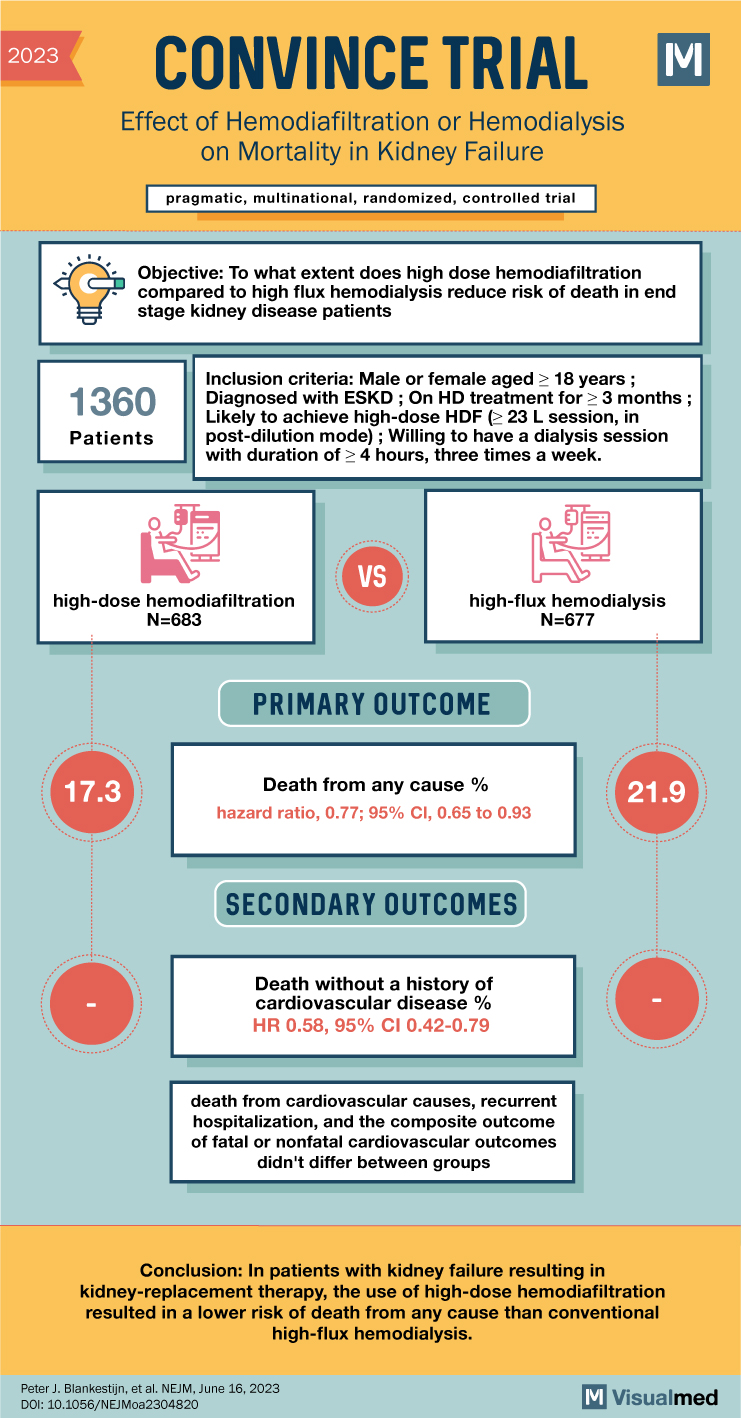
CONVINCE Trial: Hemodiafiltration or Hemodialysis in Kidney Failure
CONVINCE Trial Summary The abstract discusses the results of the CONVINCE trial, a randomized, controlled study comparing high-dose hemodiafiltration (HDF) with conventional high-flux hemodialysis (HD) in patients with end-stage kidney disease (ESKD). The trial aimed to determine whether high-dose HDF … Read More
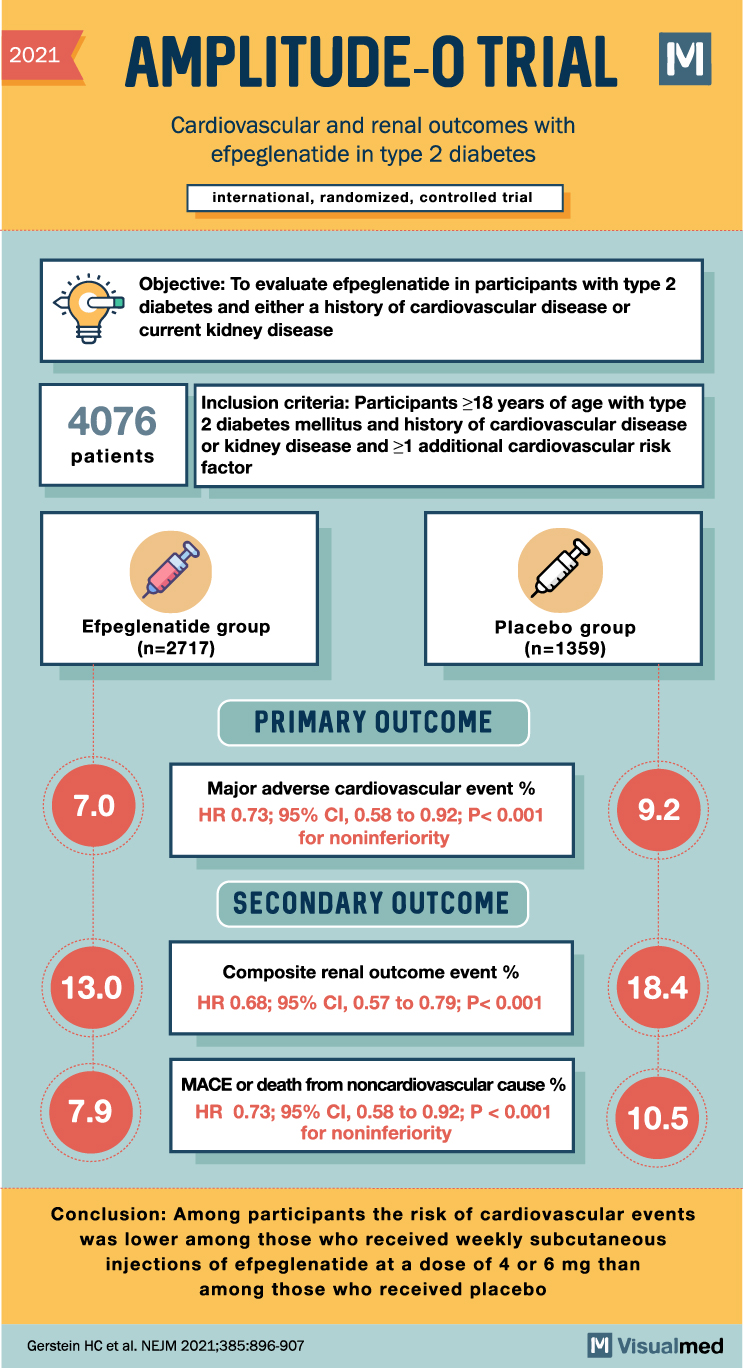
AMPLITUDE-O Trial: CV and Renal Outcomes with Efpeglenatide
2021 AMPLITUDE-O TRIAL M Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes international, randomized, controlled trial Objective: To evaluate efpeglenatide in participants with type 2 diabetes and either a history of cardiovascular disease or current kidney disease 4076 … Read More
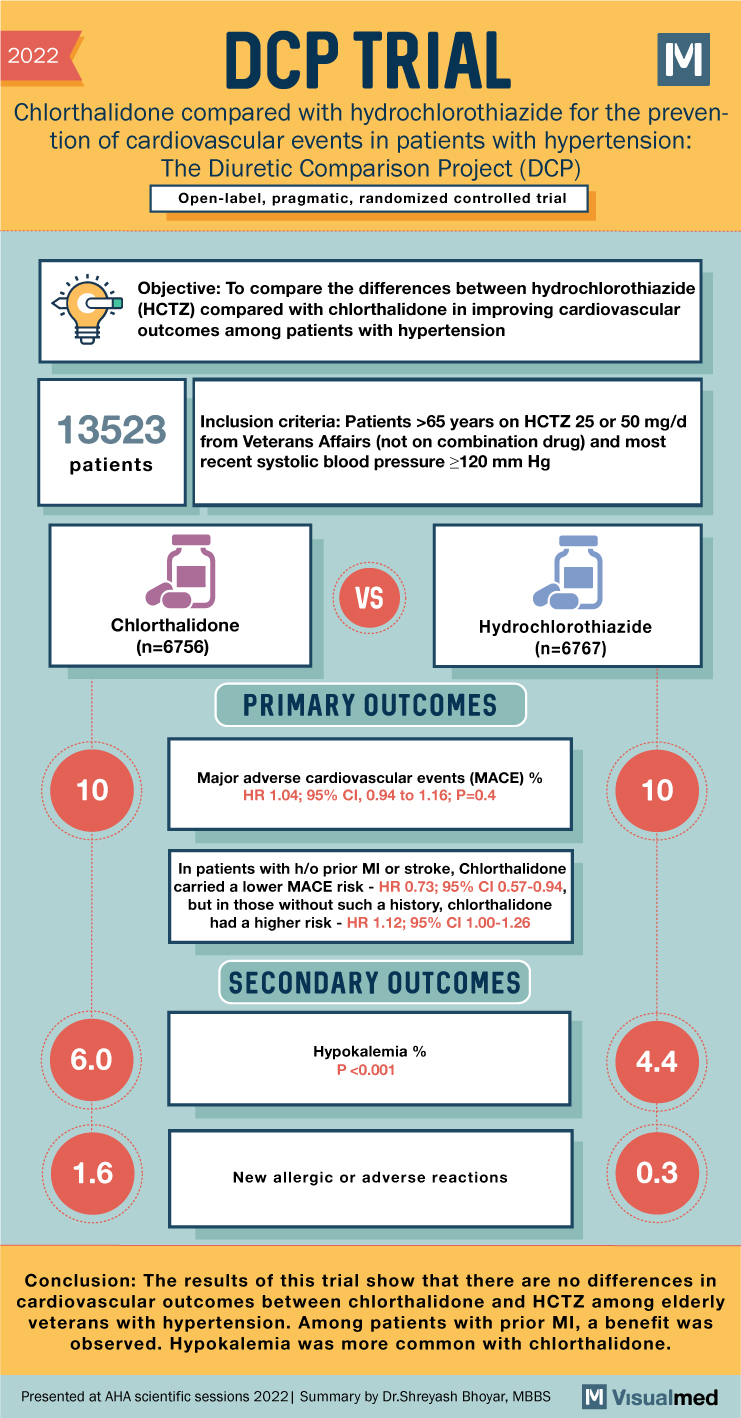
DCP Trial Summary: HCTZ vs Chlorthalidone
2022 DCP TRIAL Chlorthalidone compared with hydrochlorothiazide for the preven- tion of cardiovascular events in patients with hypertension: The Diuretic Comparison Project (DCP) Open-label, pragmatic, randomized controlled trial Objective: To compare the differences between hydrochlorothiazide (HCTZ) compared with chlorthalidone in … Read More
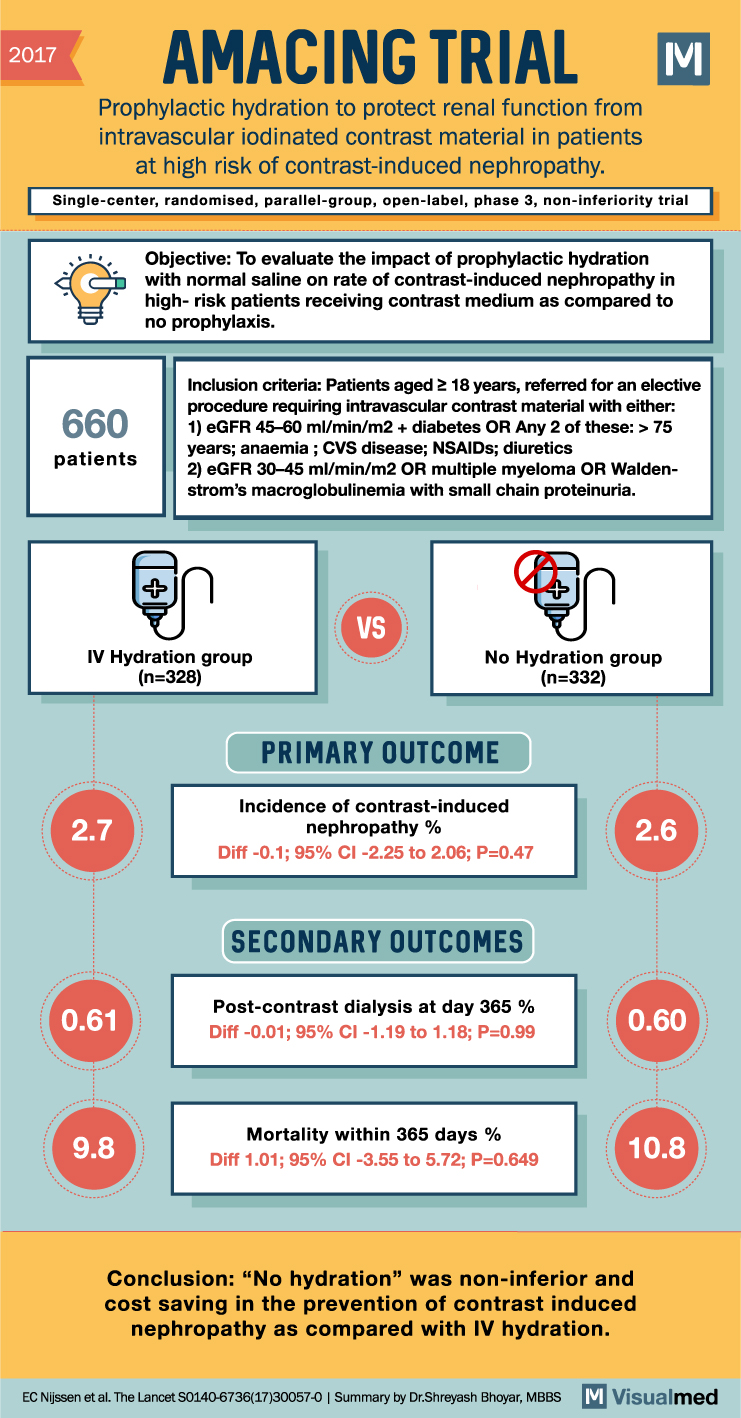
AMACING Trial Summary: IV Hydration for CIN
2017 AMACING TRIAL Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy. M Single-center, randomised, parallel-group, open-label, phase 3, non-inferiority trial 660 patients Objective: To evaluate the impact of prophylactic … Read More
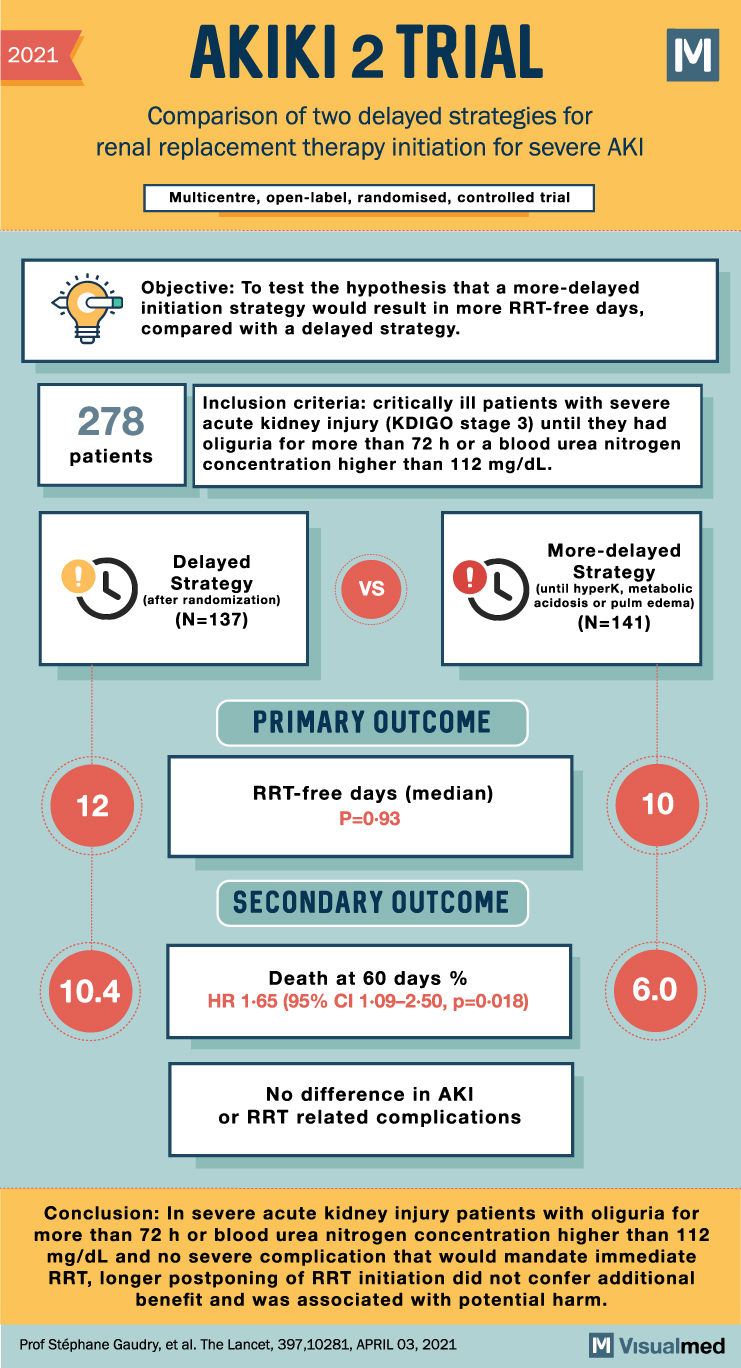
AKIKI 2 Trial Summary: RRT for Severe AKI
2021 AKIKI 2 TRIAL Comparison of two delayed strategies for renal replacement therapy initiation for severe AKI Multicentre, open-label, randomised, controlled trial Objective: To test the hypothesis that a more-delayed initiation strategy would result in more RRT-free days, compared with … Read More
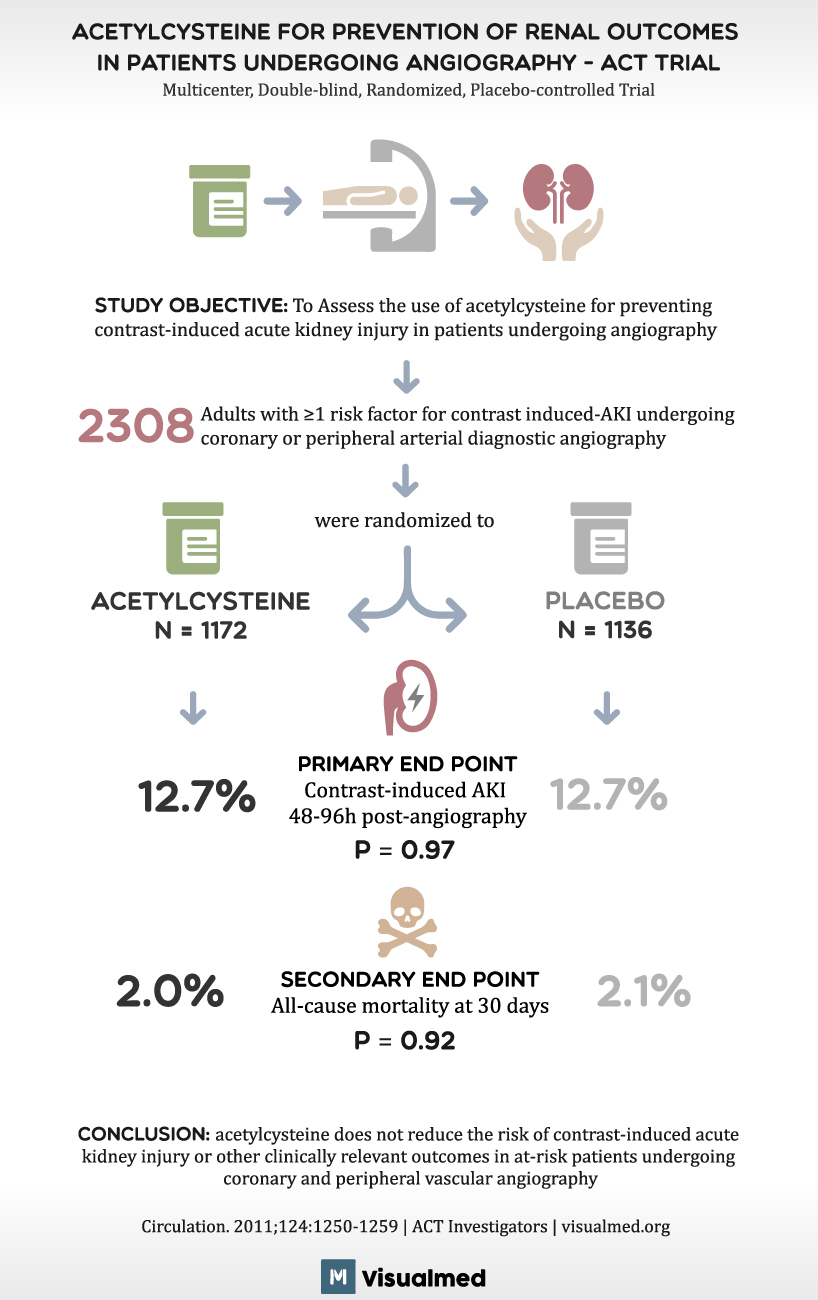
ACT Trial Summary: Acetylcysteine before Angiography
ACETYLCYSTEINE FOR PREVENTION OF RENAL OUTCOMES IN PATIENTS UNDERGOING ANGIOGRAPHY – ACT TRIAL Multicenter, Double-blind, Randomized, Placebo-controlled Trial STUDY OBJECTIVE: To Assess the use of acetylcysteine for preventing contrast-induced acute kidney injury in patients undergoing angiography 2308 Adults with ≥1 … Read More
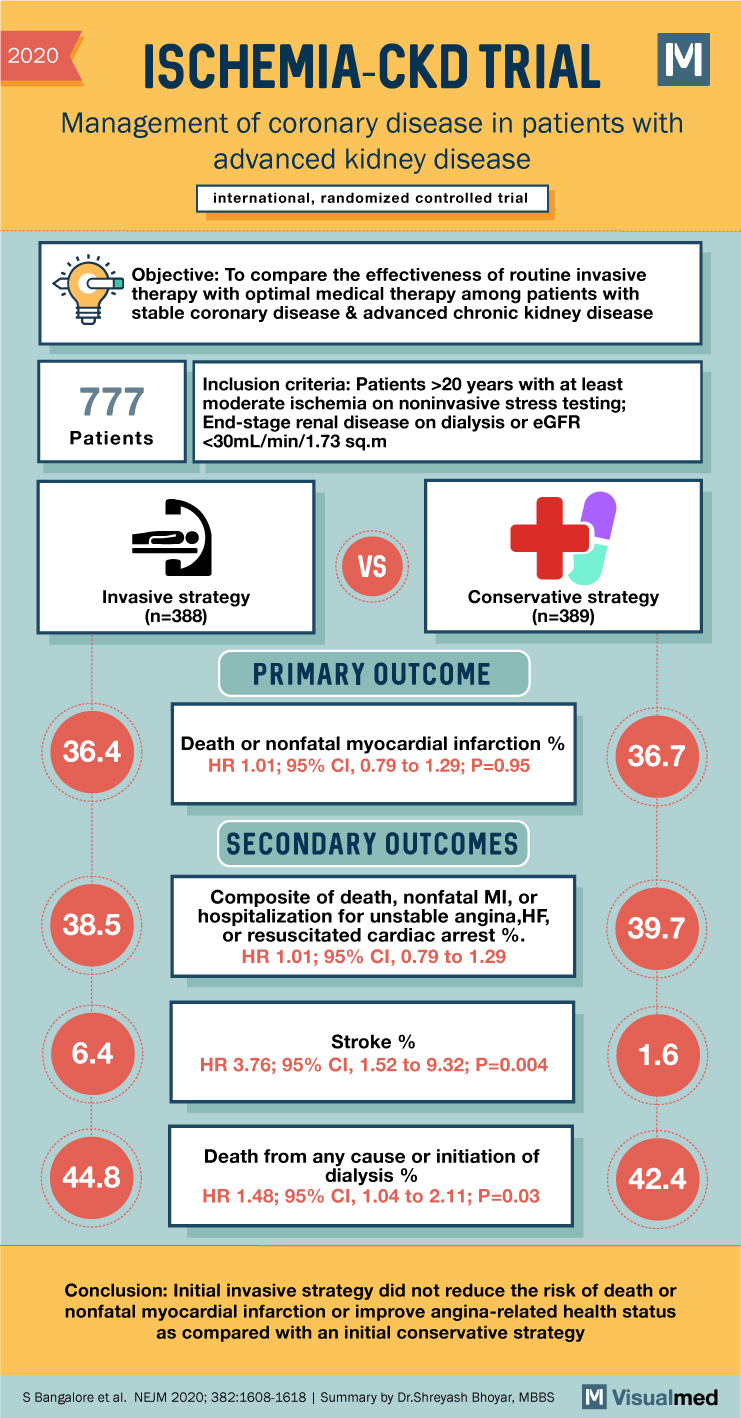
ISCHEMIA-CKD Trial Summary: CAD Management in Advanced CKD
2020 ISCHEMIA-CKD TRIAL Management of coronary disease in patients with advanced kidney disease international, randomized controlled trial Objective: To compare the effectiveness of routine invasive therapy with optimal medical therapy among patients with stable coronary disease & advanced chronic kidney … Read More
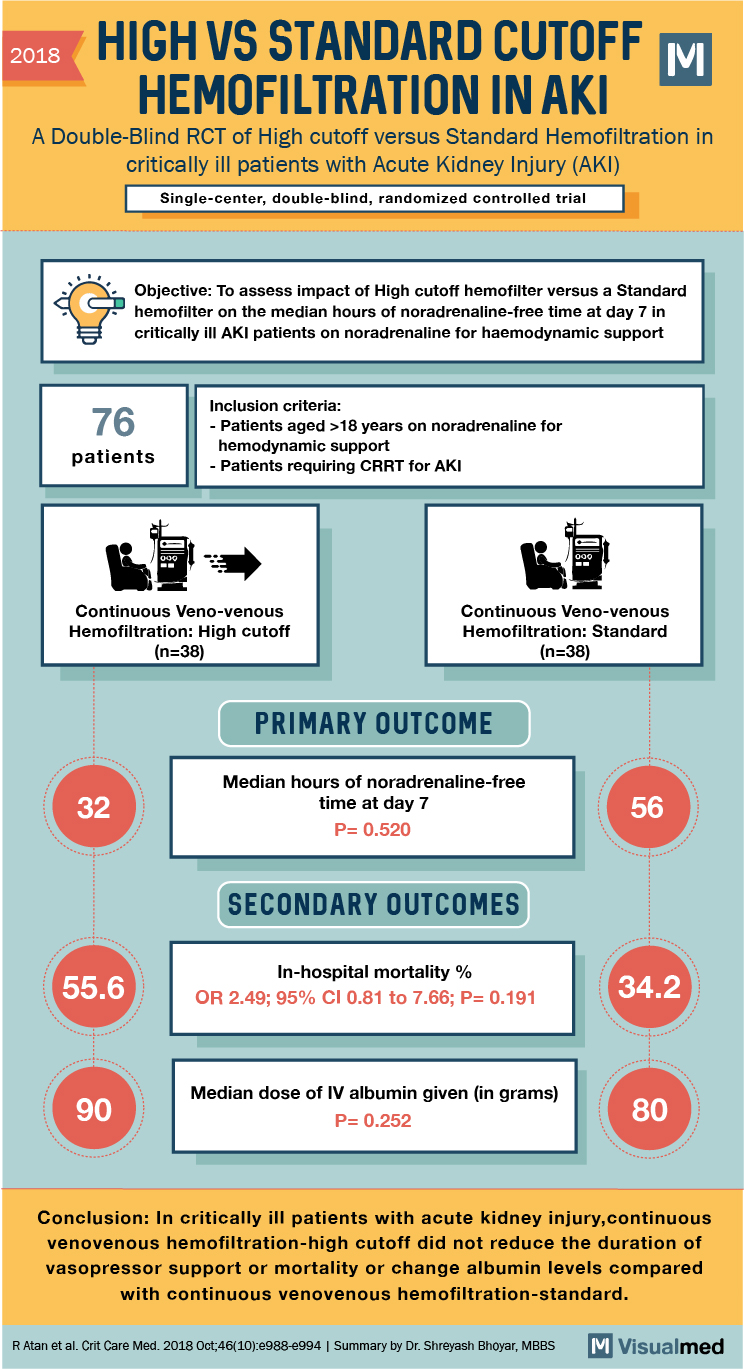
Cut-off Haemofiltration With Standard Haemofiltration in Acute Renal Failure
2018 HIGH VS STANDARD CUTOFF HEMOFILTRATION IN AKI A Double-Blind RCT of High cutoff versus Standard Hemofiltration in critically ill patients with Acute Kidney Injury (AKI) Single-center, double-blind, randomized controlled trial Objective: To assess impact of High cutoff hemofilter versus … Read More