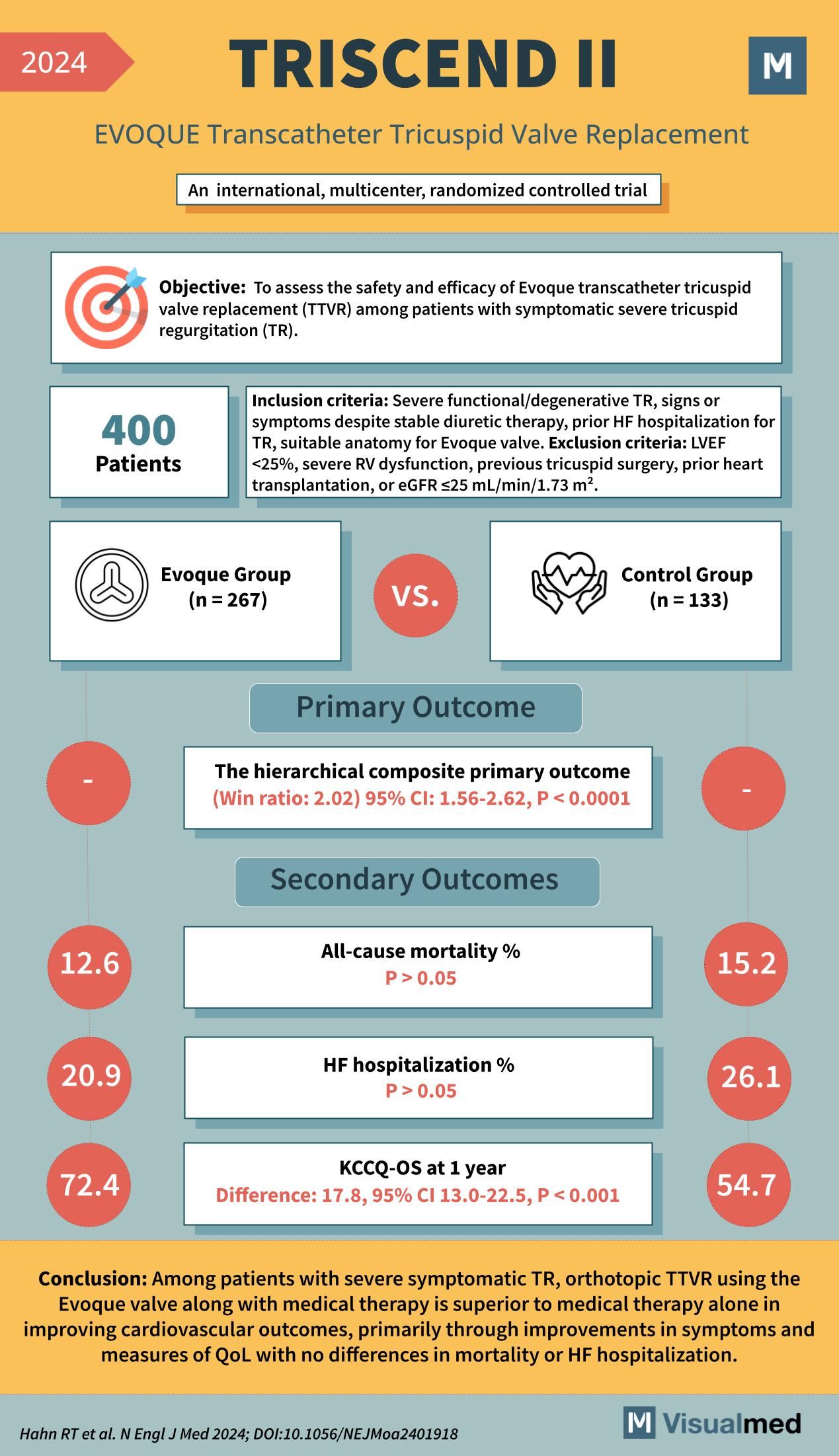
TRISCEND II Trial: EVOQUE Transcatheter Tricuspid Valve Replacement (TTVR)
Severe tricuspid regurgitation (TR) has historically been challenging to treat, with medical therapy offering limited relief. The TRISCEND II trial (2024) marks a significant milestone by assessing the safety and efficacy of the EVOQUE transcatheter tricuspid valve replacement (TTVR) in patients with symptomatic severe TR.
Study Overview
The TRISCEND II trial was an international, multicenter, randomized controlled trial designed to evaluate EVOQUE TTVR versus medical therapy alone.
Objective
To determine whether EVOQUE TTVR improves clinical outcomes in patients with symptomatic severe TR.
Participants
- 400 patients randomized to:
- EVOQUE group (n=267)
- Control group (n=133)
Inclusion Criteria
Patients with:
- Severe functional/degenerative TR
- Persistent symptoms despite stable diuretic therapy
- History of heart failure (HF) hospitalization for TR
- Suitable anatomy for EVOQUE valve
Exclusion Criteria
- Left ventricular ejection fraction (LVEF) <25%
- Severe right ventricular dysfunction
- Previous tricuspid valve surgery
- Prior heart transplantation
- eGFR ≤25 mL/min/1.73 m²
Key Outcomes
Primary Outcome
The hierarchical composite primary outcome:
- Win ratio = 2.02
- 95% CI: 1.56–2.62, P < 0.0001
EVOQUE TTVR was superior to medical therapy alone, as demonstrated by the significant win ratio.
Secondary Outcomes
- All-cause mortality:
- EVOQUE: 12.6% vs. Control: 15.2%
- No significant difference (P > 0.05)
- Heart failure hospitalization:
- EVOQUE: 20.9% vs. Control: 26.1%
- No significant difference (P > 0.05)
- Quality of life (QoL) – KCCQ-OS at 1 year:
- Significant improvement with EVOQUE:
- 72.4 vs. Control: 54.7
- Difference: 17.8 points, 95% CI: 13.0–22.5, P < 0.001
- Significant improvement with EVOQUE:
Conclusion
The TRISCEND II trial highlights the potential of EVOQUE TTVR as a transformative therapy for patients with symptomatic severe TR. Compared to medical therapy alone, EVOQUE TTVR demonstrated:
- Superior improvement in symptoms and QoL
- Comparable rates of mortality and HF hospitalization
This trial paves the way for broader adoption of transcatheter therapies in managing TR, offering hope to patients who previously had limited treatment options.
References
Hahn RT et al. “TRISCEND II: EVOQUE Transcatheter Tricuspid Valve Replacement.” N Engl J Med 2024. DOI: 10.1056/NEJMoa2401918.