The COVID-19 pandemic triggered an unprecedented surge in clinical research, leading to thousands of studies aimed at understanding, managing, and ultimately controlling the virus. As healthcare systems worldwide faced the immense challenge of treating patients, researchers raced against time to … Read More
anticoagulation
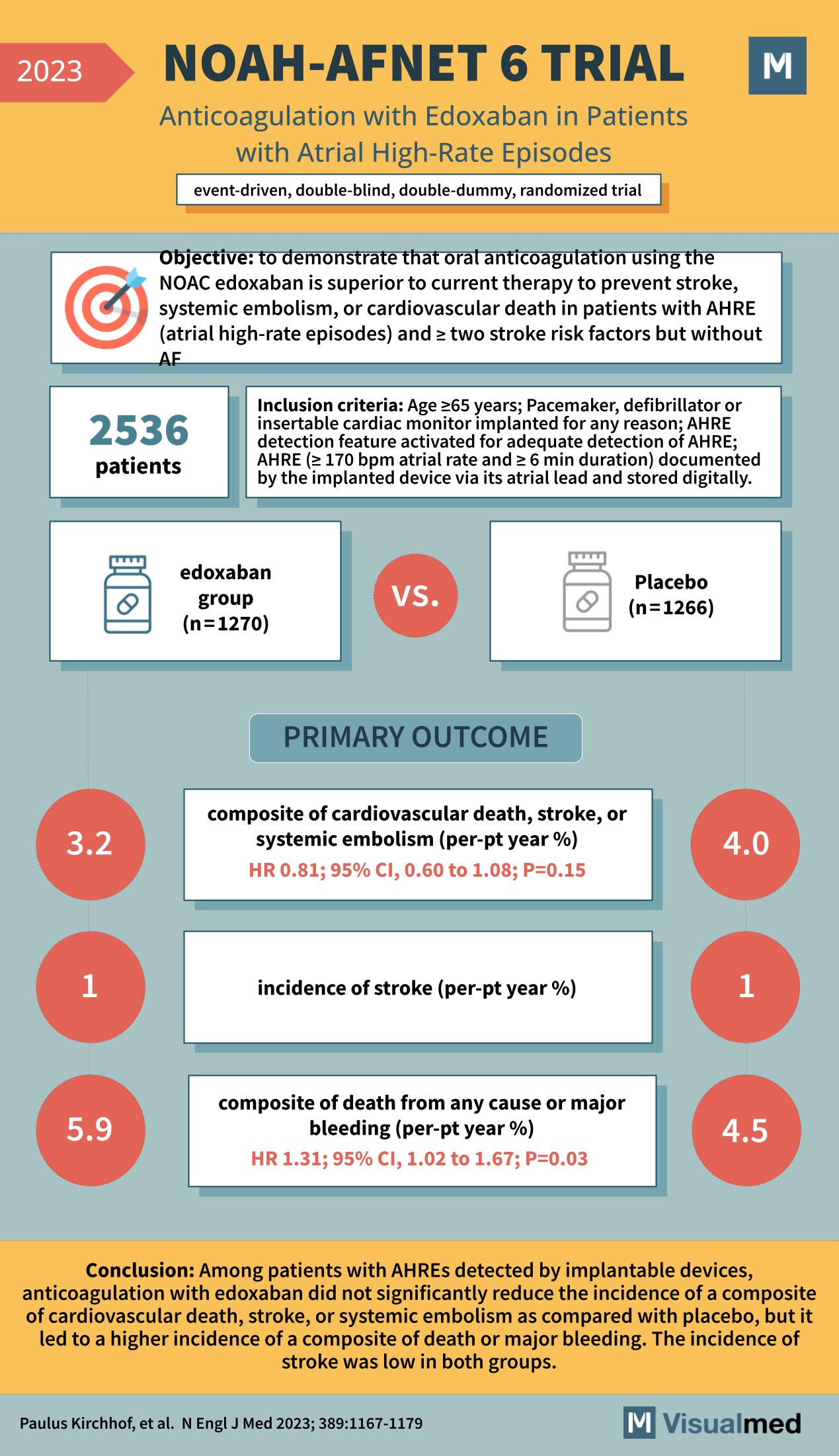
NOAH-AFNET 6 Trial: Edoxaban in AHRE
The NOAH-AFNET 6 trial, as outlined in a groundbreaking study from the New England Journal of Medicine in 2023, represents a significant advancement in our understanding of atrial fibrillation management. This study scrutinized the efficacy of edoxaban, a novel oral … Read More
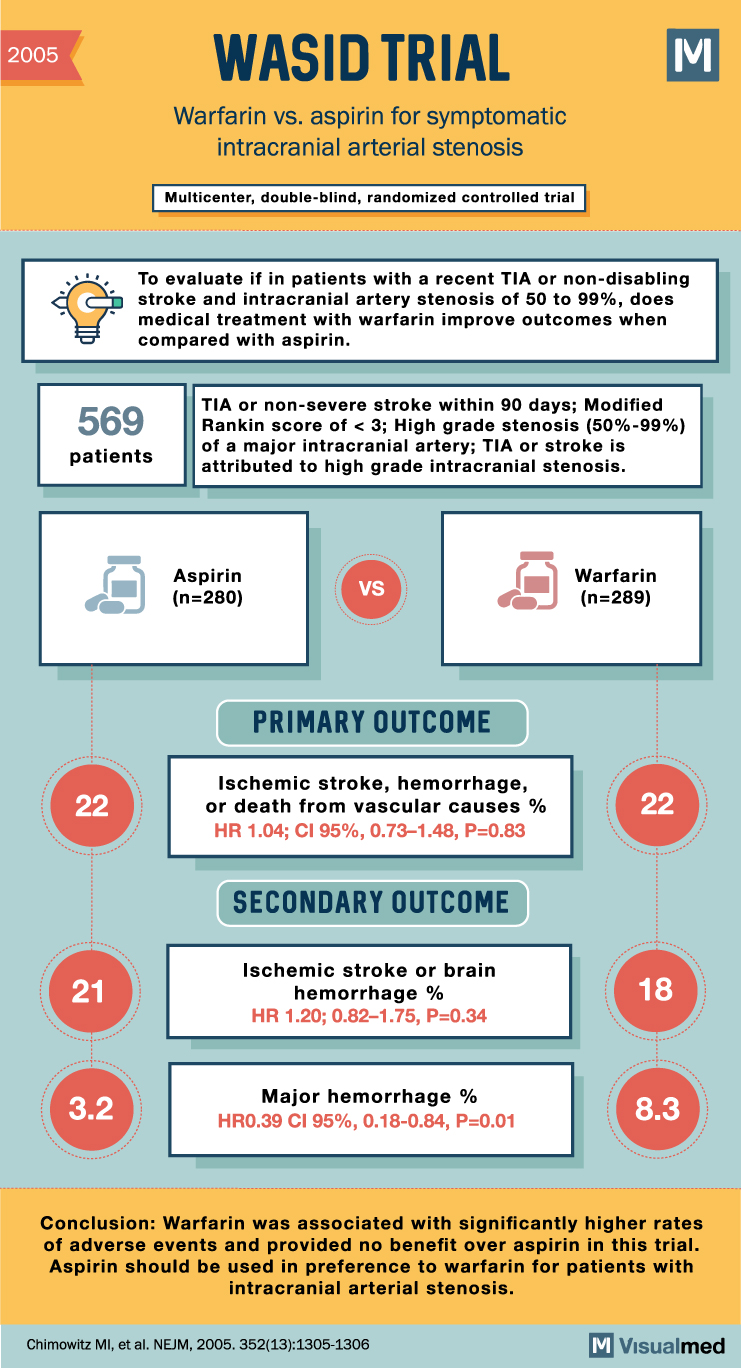
WASID Trial Summary: Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis
WASID Trial Summary The WASID trial sought to compare the effectiveness of warfarin and aspirin in the treatment of atherosclerotic intracranial arterial stenosis, a significant cause of stroke. While warfarin is often preferred over aspirin for this condition, the two … Read More
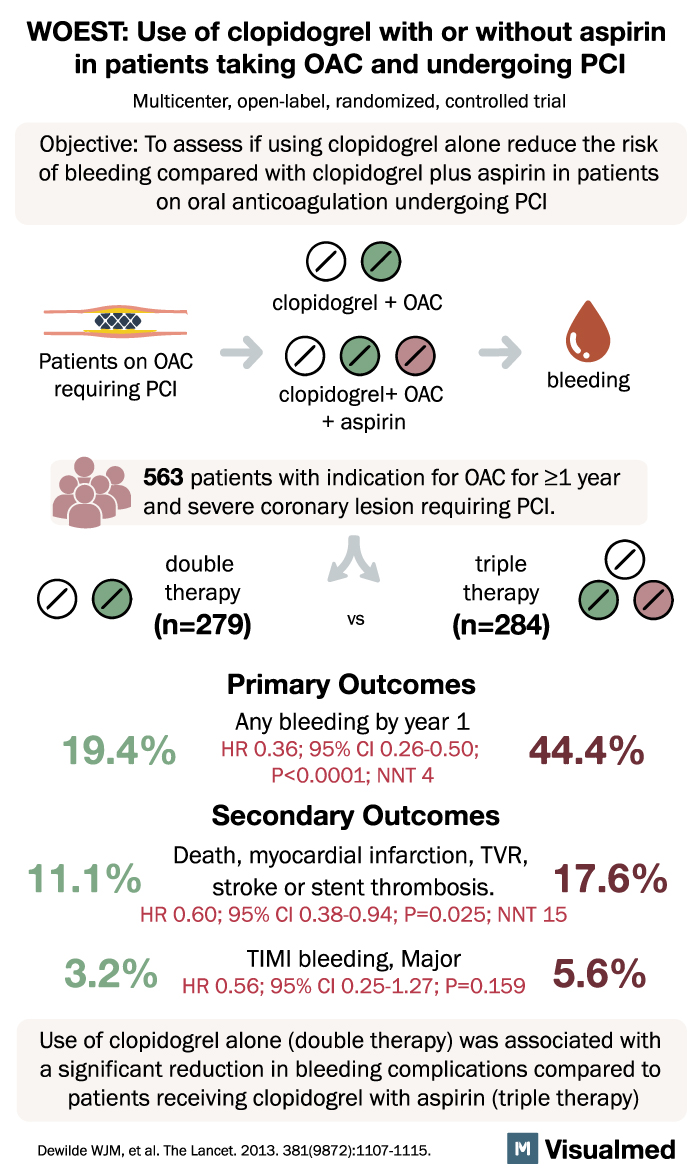
WOEST Trial Summary: Clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing PCI
WOEST Trial Summary The WOEST trial, a groundbreaking study published in The Lancet, has shed light on a significant issue faced by patients undergoing percutaneous coronary intervention (PCI) while taking oral anticoagulants. Traditionally, triple therapy involving aspirin and clopidogrel has … Read More
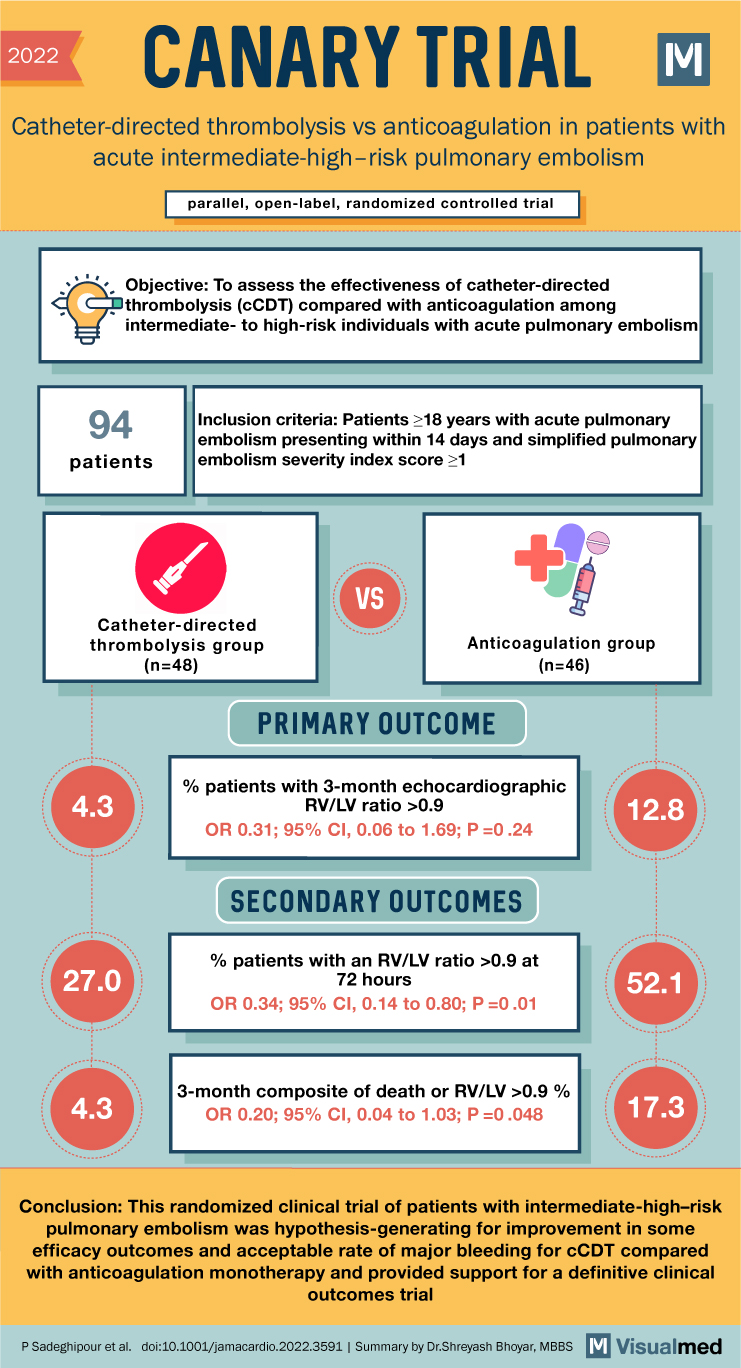
CANARY Trial Summary: Cath vs AC in PE
2022 CANARY TRIAL Catheter-directed thrombolysis vs anticoagulation in patients with acute intermediate-high-risk pulmonary embolism parallel, open-label, randomized controlled trial Objective: To assess the effectiveness of catheter-directed thrombolysis (CCDT) compared with anticoagulation among intermediate- to high-risk individuals with acute pulmonary embolism … Read More
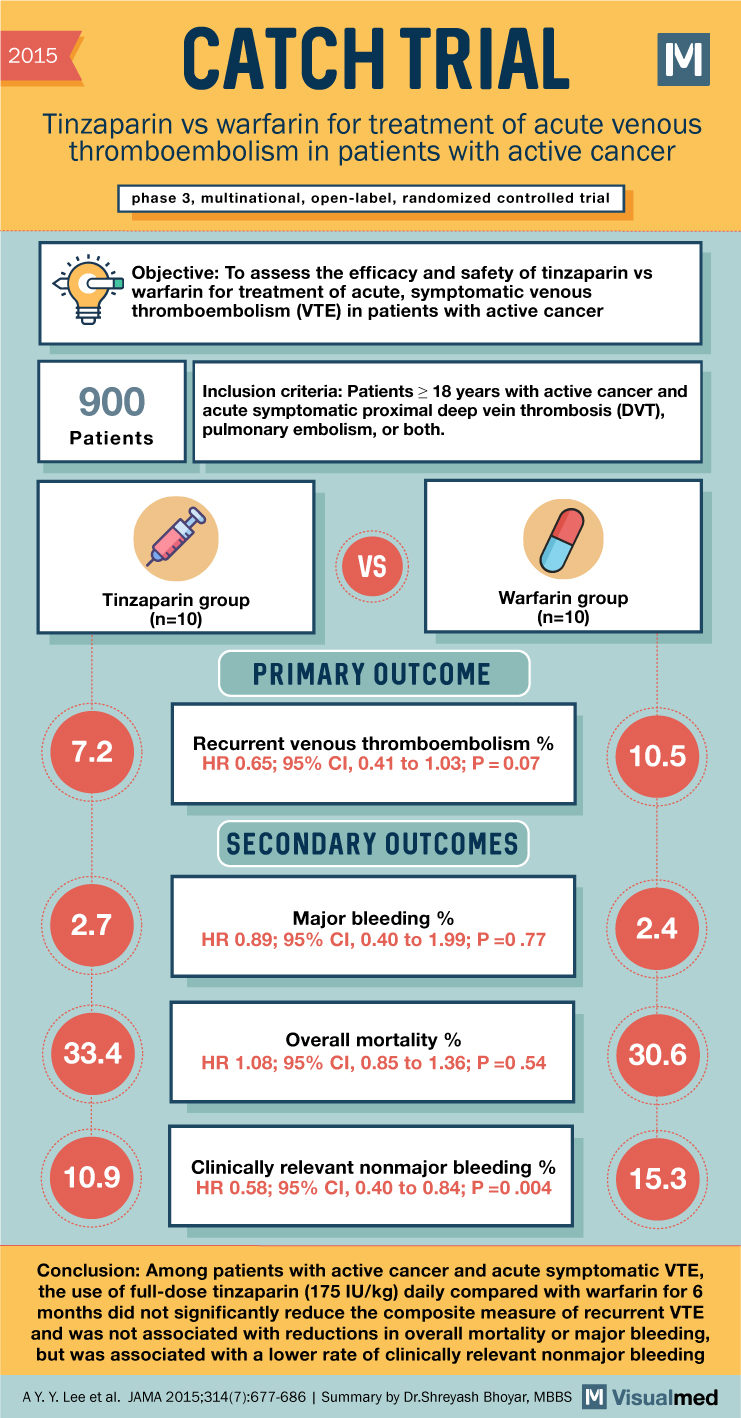
CATCH Trial Summary: Tinzaparin for VTE in Cancer
2015 CATCH TRIAL Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer phase 3, multinational, open-label, randomized controlled trial Objective: To assess the efficacy and safety of tinzaparin vs warfarin for treatment of acute, symptomatic … Read More
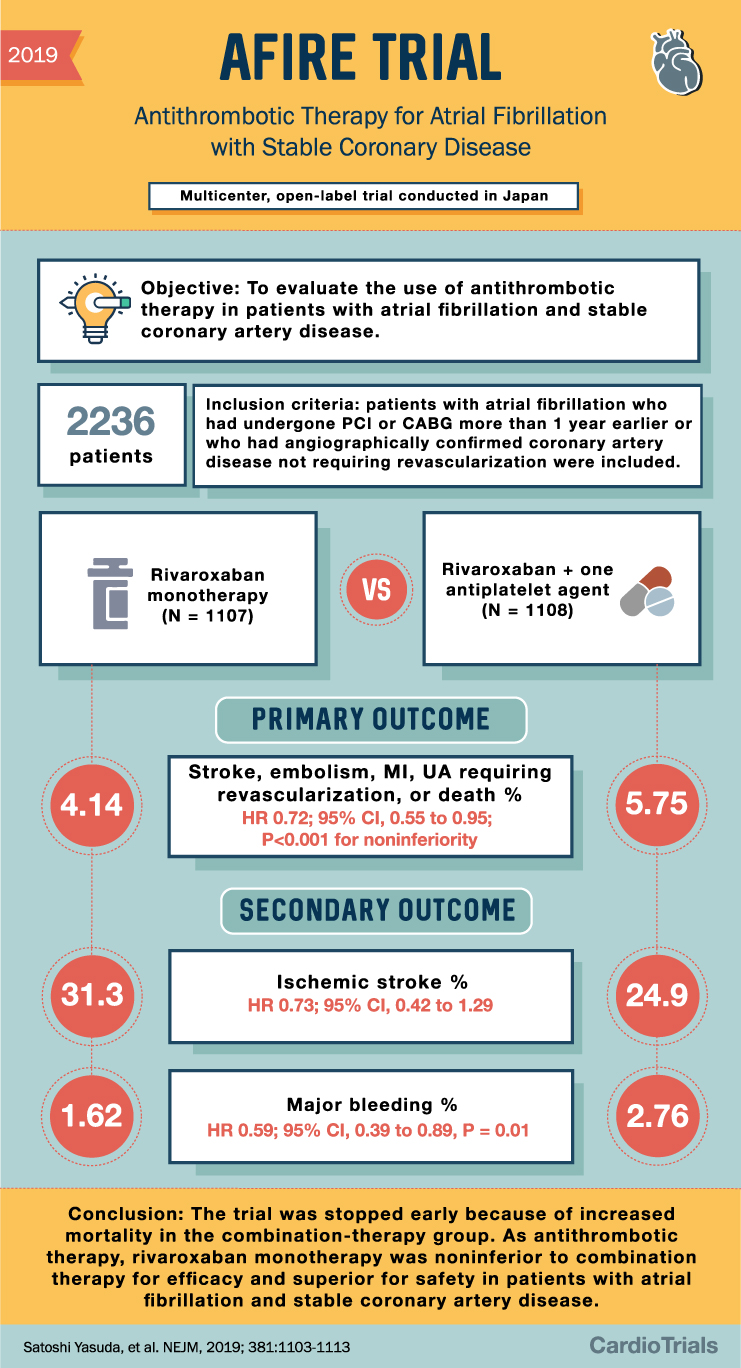
AFIRE Trial Summary: Antithrombotics in AFib and CAD
2019 AFIRE TRIAL Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease Multicenter, open-label trial conducted in Japan Objective: To evaluate the use of antithrombotic therapy in patients with atrial fibrillation and stable coronary artery disease. 2236 patients 4.14 Inclusion … Read More
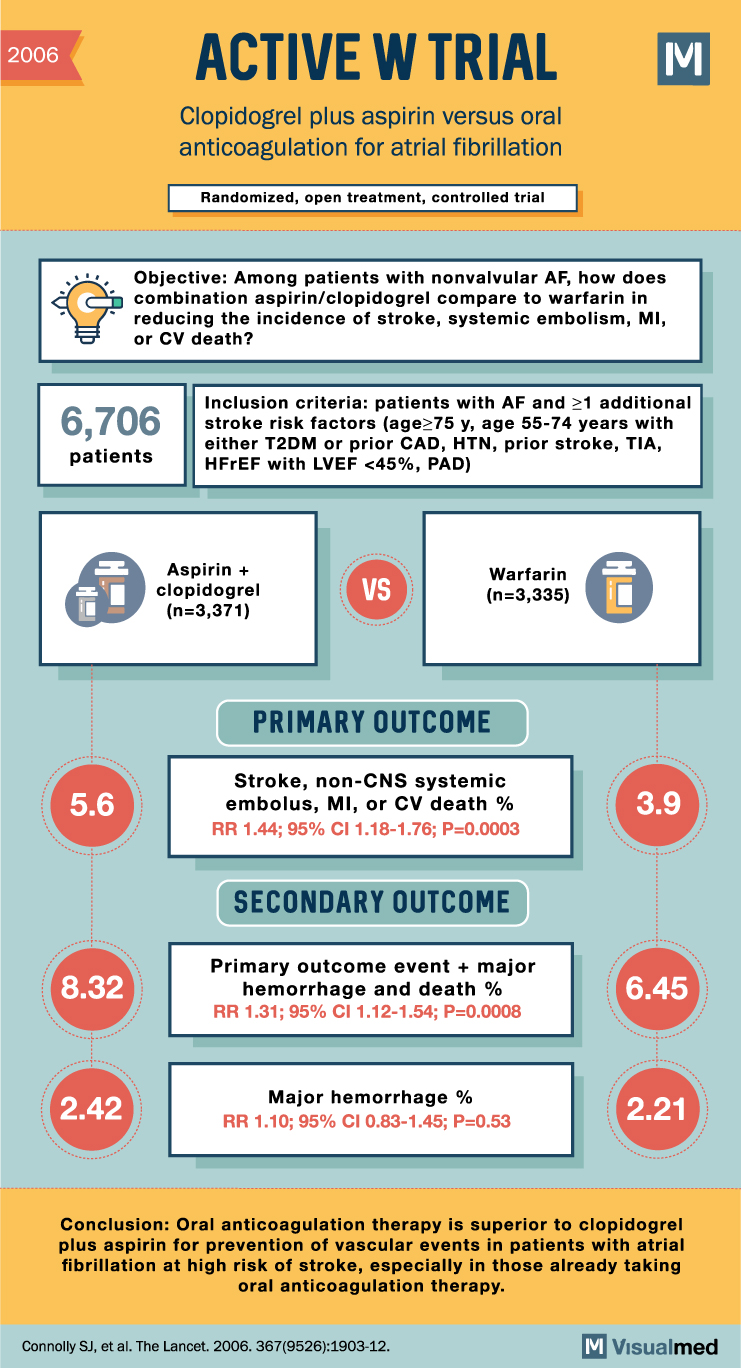
ACTIVE W Trial Summary: Clopidogrel + ASA vs. OAC for Atrial Fibrillation
2006 ACTIVE W TRIAL Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation Randomized, open treatment, controlled trial M Objective: Among patients with nonvalvular AF, how does combination aspirin/clopidogrel compare to warfarin in reducing the incidence of stroke, systemic embolism, … Read More
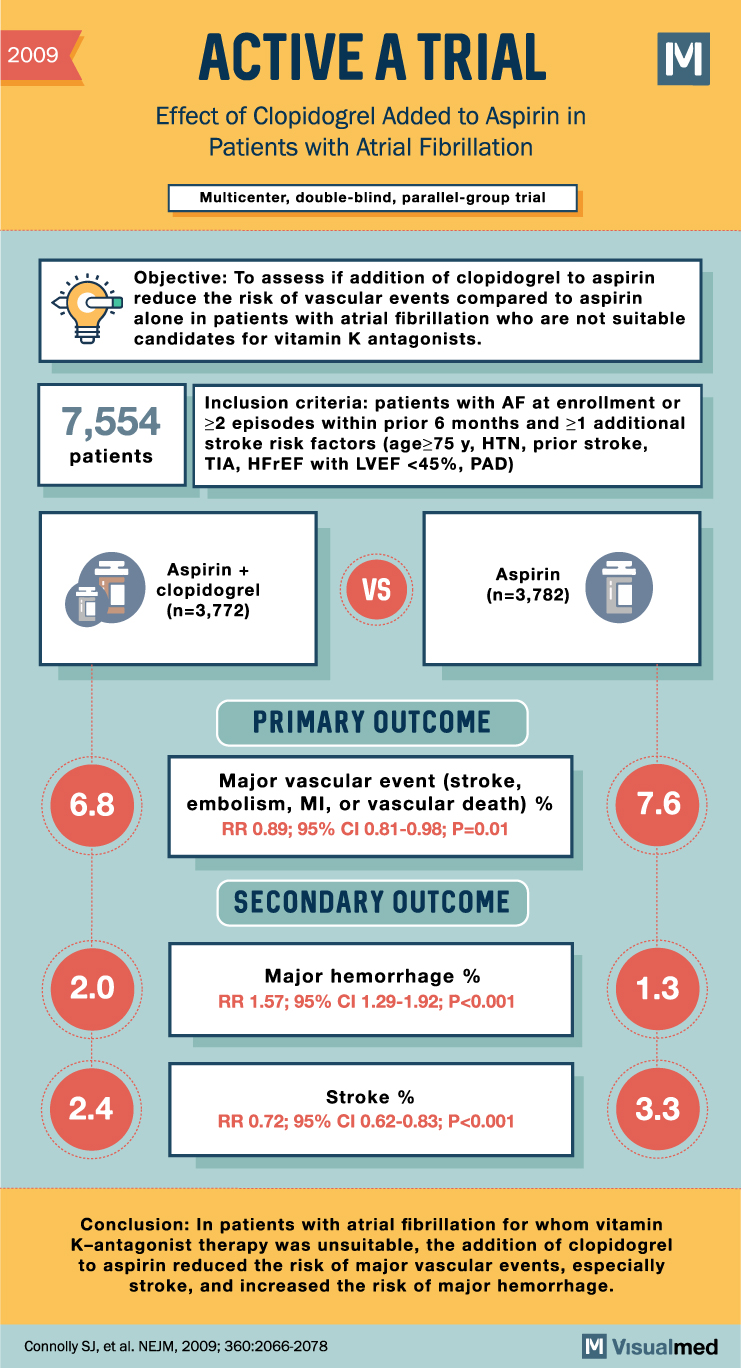
ACTIVE A Trial Summary: Clopidogrel + Aspirin in Atrial Fibrillation
2009 ACTIVE A TRIAL Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation Multicenter, double-blind, parallel-group trial M Objective: To assess if the addition of clopidogrel to aspirin reduces the risk of vascular events compared to aspirin alone … Read More
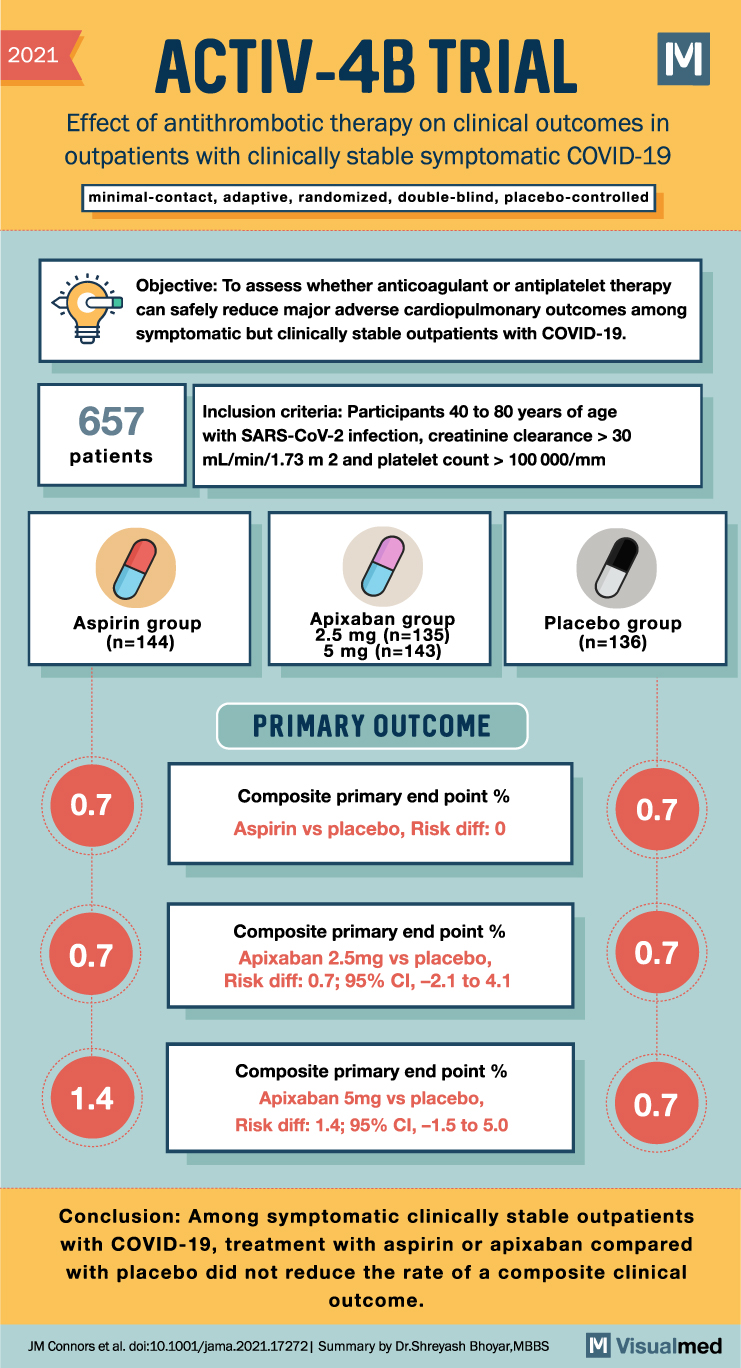
ACTIV-4B Trial Summary: Antithrombotic Therapy in Covid-19
2021 ACTIV-4B TRIAL Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19 minimal-contact, adaptive, randomized, double-blind, placebo-controlled Objective: To assess whether anticoagulant or antiplatelet therapy can safely reduce major adverse cardiopulmonary outcomes among symptomatic but … Read More
