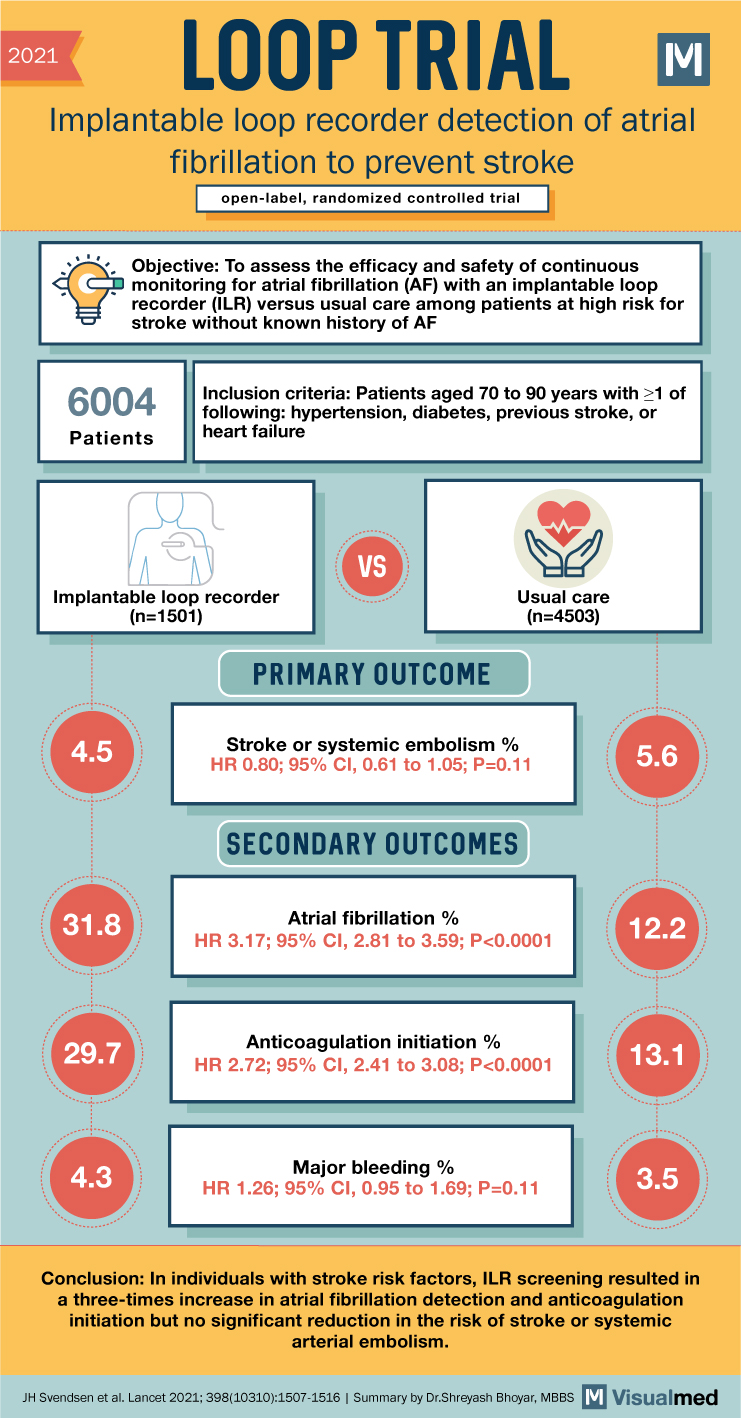
2021 LOOP TRIAL Implantable loop recorder detection of atrial fibrillation to prevent stroke open-label, randomized controlled trial Objective: To assess the efficacy and safety of continuous monitoring for atrial fibrillation (AF) with an implantable loop recorder (ILR) versus usual care among patients at high risk for stroke without known history of AF 6004 Patients Inclusion criteria: Patients aged 70 to 90 years with >1 of following: hypertension, diabetes, previous stroke, or heart failure Implantable loop recorder (n=1501) 4.5 VS Usual care (n=4503) PRIMARY OUTCOME Stroke or systemic embolism% HR 0.80; 95% CI, 0.61 to 1.05; P=0.11 SECONDARY OUTCOMES 5.6 31.8 Atrial fibrillation % 12.2 HR 3.17; 95% CI, 2.81 to 3.59; P<0.0001 Anticoagulation initiation % 29.7 13.1 HR 2.72; 95% CI, 2.41 to 3.08; P<0.0001 4.3 Major bleeding % HR 1.26; 95% CI, 0.95 to 1.69; P=0.11 3.5 Conclusion: In individuals with stroke risk factors, ILR screening resulted in a three-times increase in atrial fibrillation detection and anticoagulation initiation but no significant reduction in the risk of stroke or systemic arterial embolism. JH Svendsen et al. Lancet 2021; 398(10310):1507-1516