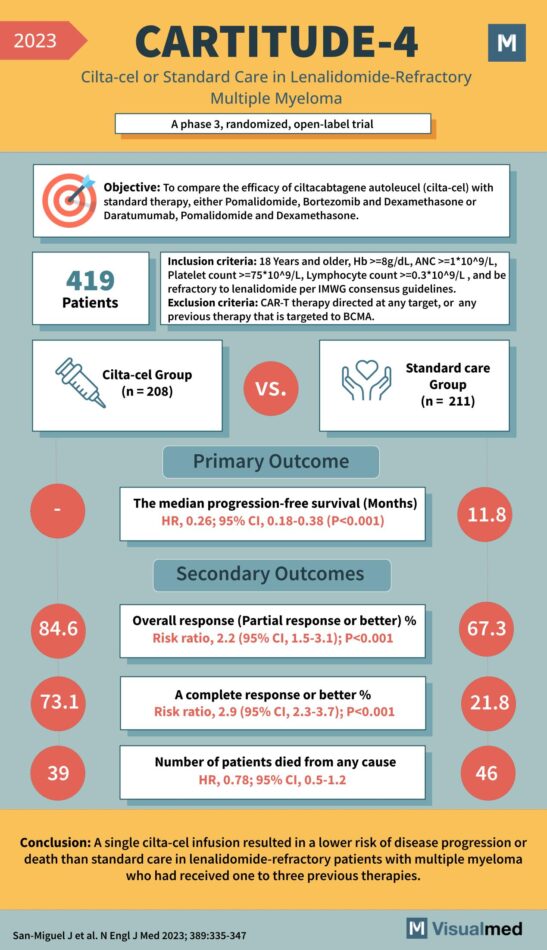
The CARTITUDE-4 Trial: Groundbreaking Treatment for Multiple Myeloma The CARTITUDE-4 Trial, a pivotal study published in 2023, represents a significant advancement in the treatment of multiple myeloma, particularly for patients with lenalidomide-refractory disease. This Phase 3 randomized, open-label trial assessed … Read More
Hematology
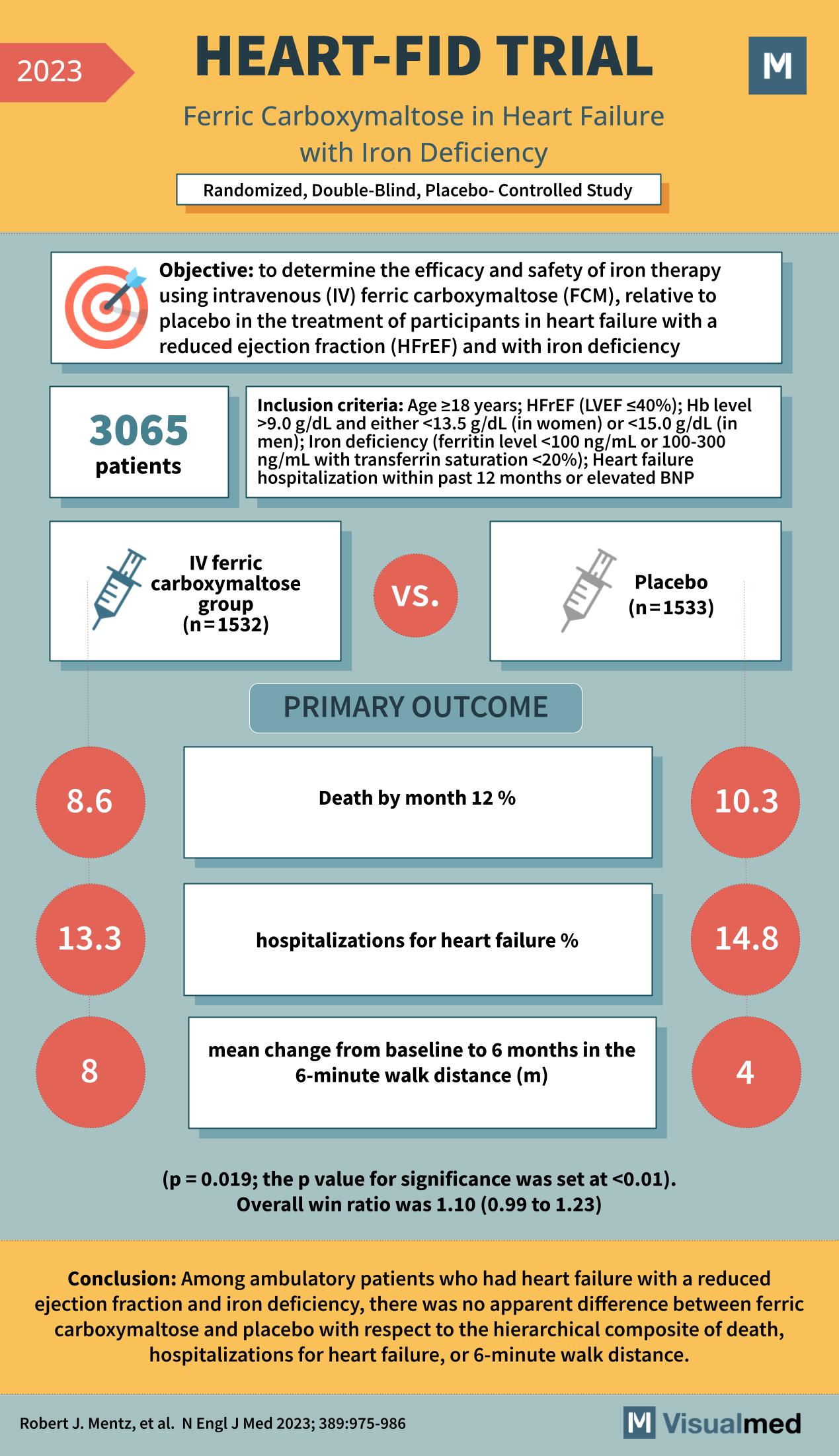
HEART-FID Trial: Iron in Heart Failure
The HEART-FID trial, as published in the New England Journal of Medicine in 2023, was a randomized, double-blind, placebo-controlled study designed to determine the efficacy and safety of intravenous ferric carboxymaltose (FCM) in treating patients with heart failure with reduced … Read More
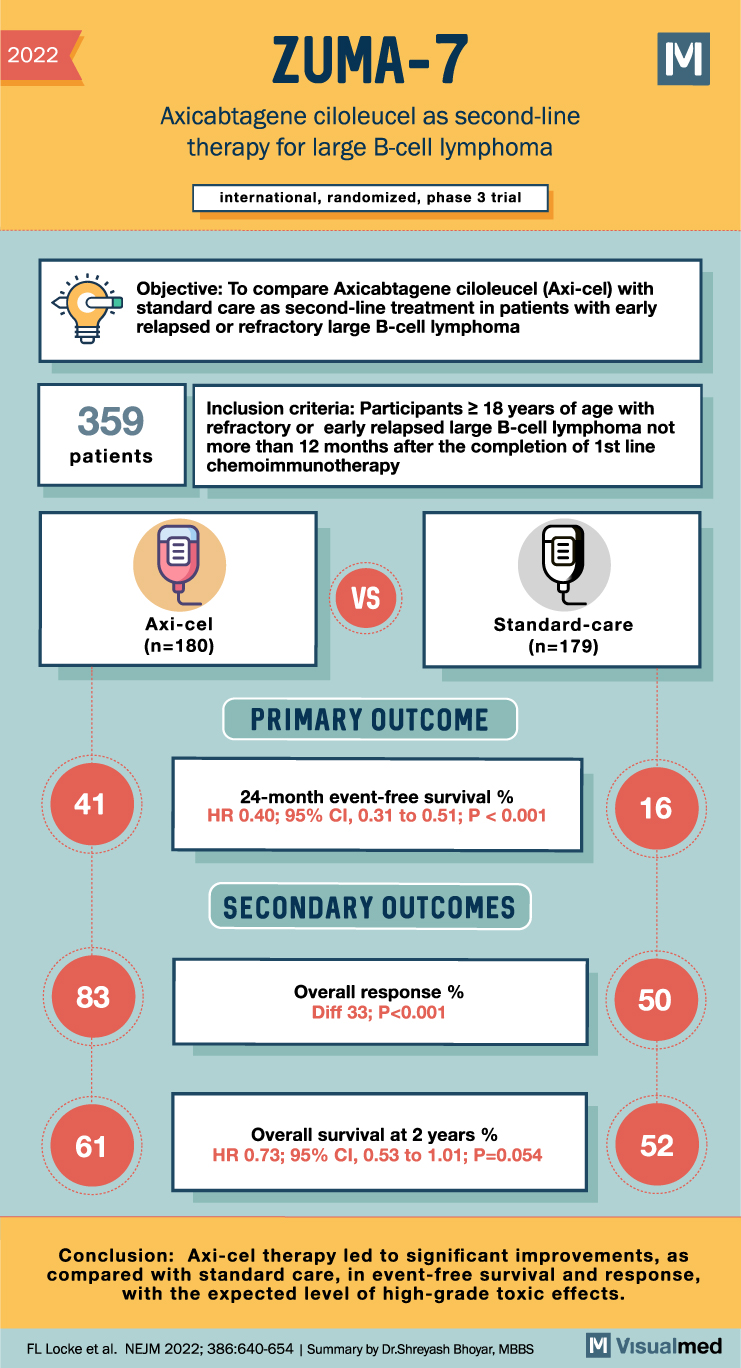
ZUMA 7 Trial: Axi-Cel Therapy- A New Hope for Patients with Early Relapsed or Refractory Large B-Cell Lymphoma
ZUMA 7 Trial Summary Patients with early relapsed or refractory large B-cell lymphoma often face poor prognoses after receiving first-line chemoimmunotherapy. However, a recent international, phase 3 trial has demonstrated promising results with the use of axicabtagene ciloleucel (axi-cel), an … Read More
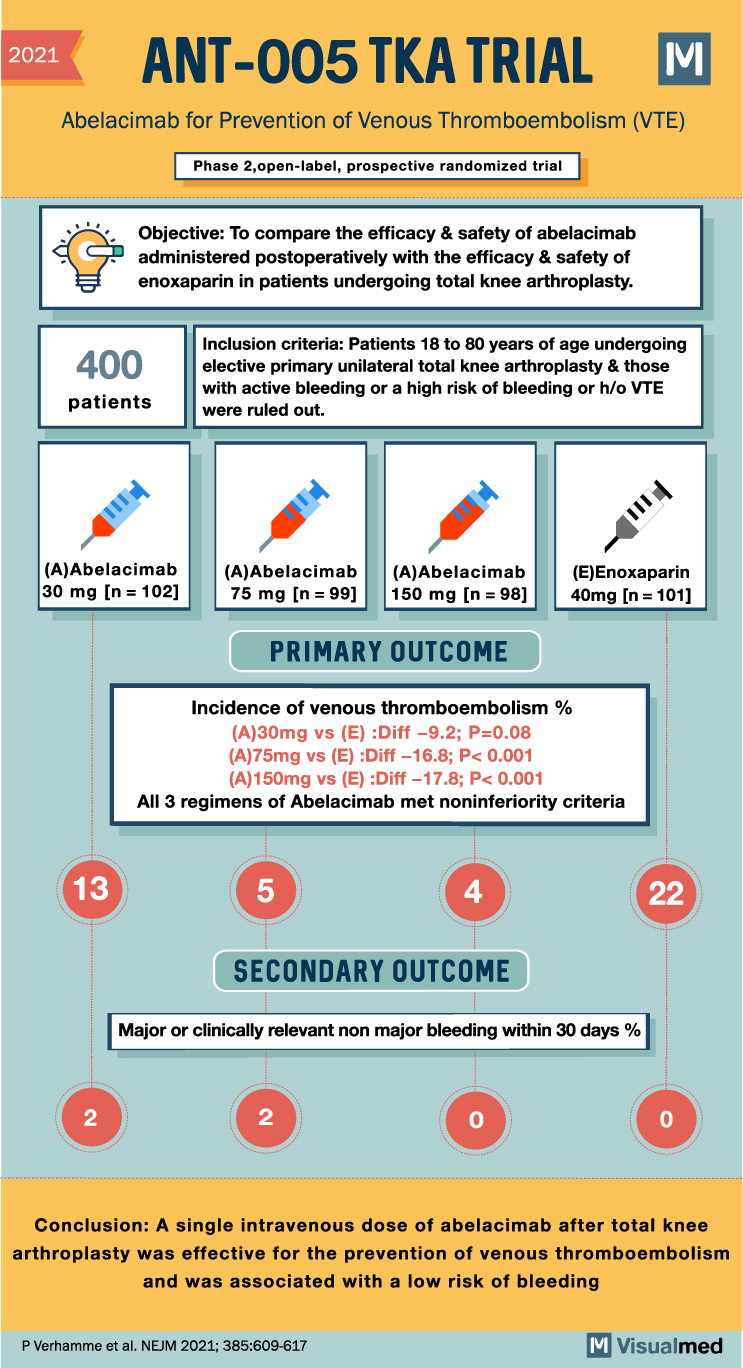
ANT 005 TKA Trial: Abelacimab for VTE
2021 ANT-005 TKA TRIAL M Abelacimab for Prevention of Venous Thromboembolism (VTE) Phase 2,open-label, prospective randomized trial 400 Objective: To compare the efficacy & safety of abelacimab administered postoperatively with the efficacy & safety of enoxaparin in patients undergoing … Read More
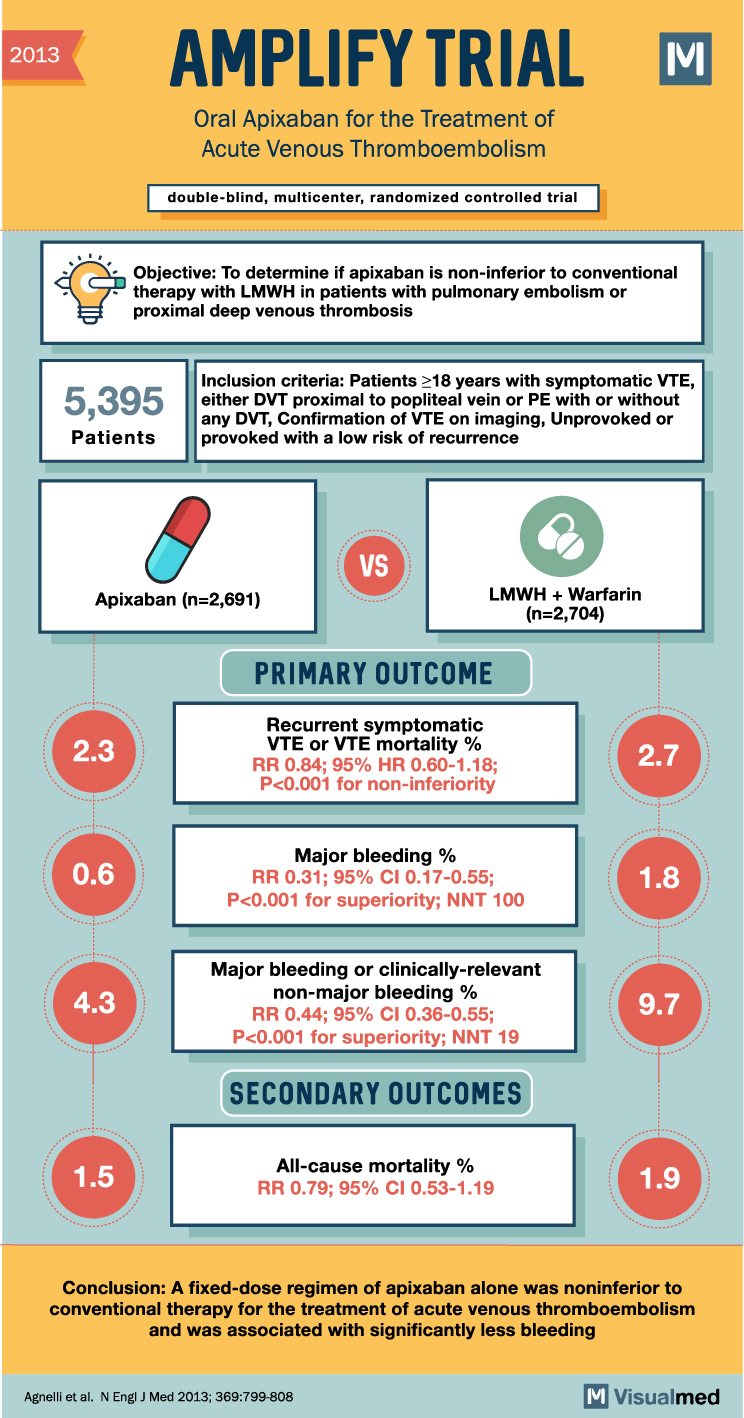
AMPLIFY Trial: Apixaban for acute VTE
2013 AMPLIFY TRIAL Oral Apixaban for the Treatment of Acute Venous Thromboembolism double-blind, multicenter, randomized controlled trial IM Objective: To determine if apixaban is non-inferior to conventional therapy with LMWH in patients with pulmonary embolism or proximal deep venous thrombosis … Read More
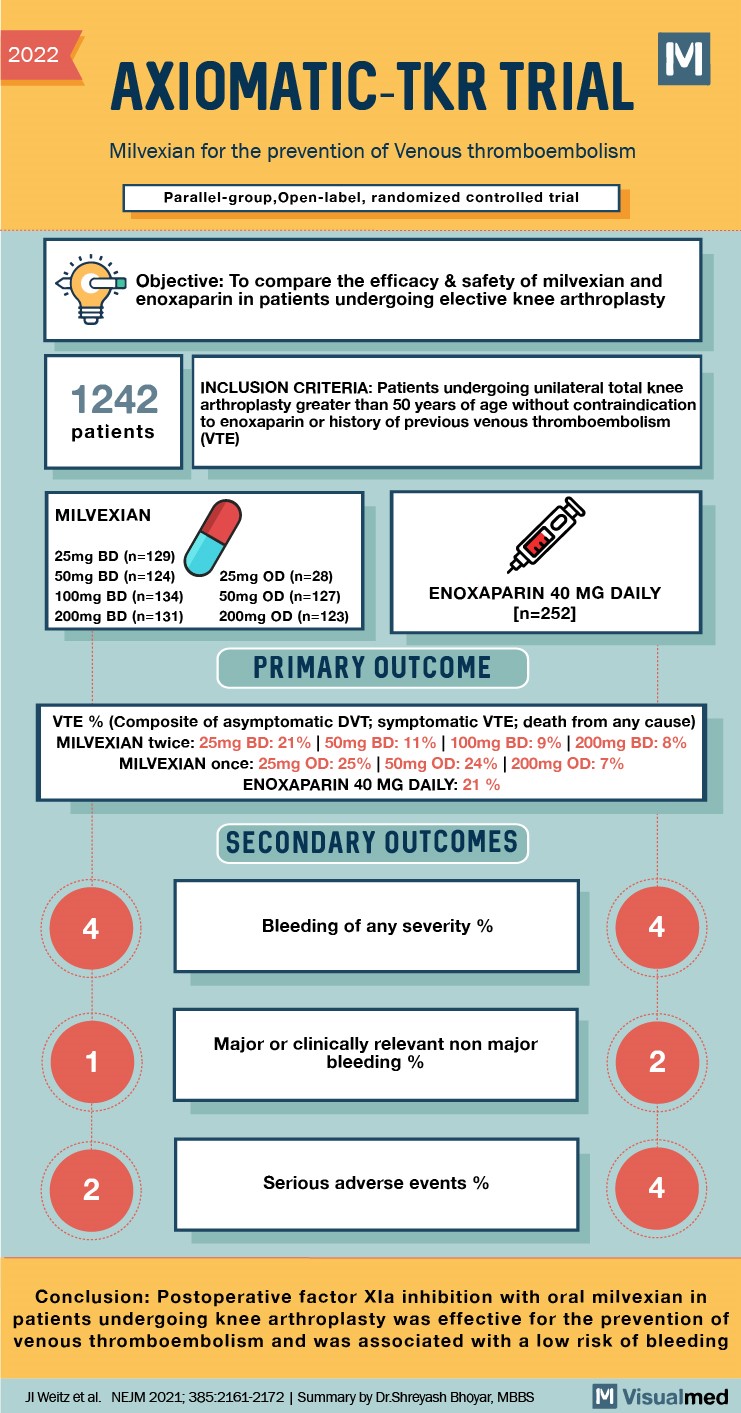
AXIOMATIC-TKR Trial Summary: Milvexian for the Prevention of VTE
2022 AXIOMATIC-TKR TRIAL Milvexian for the prevention of Venous thromboembolism Parallel-group, Open-label, randomized controlled trial Objective: To compare the efficacy & safety of milvexian and enoxaparin in patients undergoing elective knee arthroplasty 1242 patients INCLUSION CRITERIA: Patients undergoing unilateral total … Read More