Understanding the ADAPT-Sepsis Trial: PCT vs. CRP-Guided Antibiotic Duration Sepsis remains a critical challenge in intensive care units (ICUs), often requiring prolonged antibiotic use. The ADAPT-Sepsis trial, published in JAMA (2024), aimed to determine whether procalcitonin (PCT)-guided or C-reactive protein … Read More
Critical Care
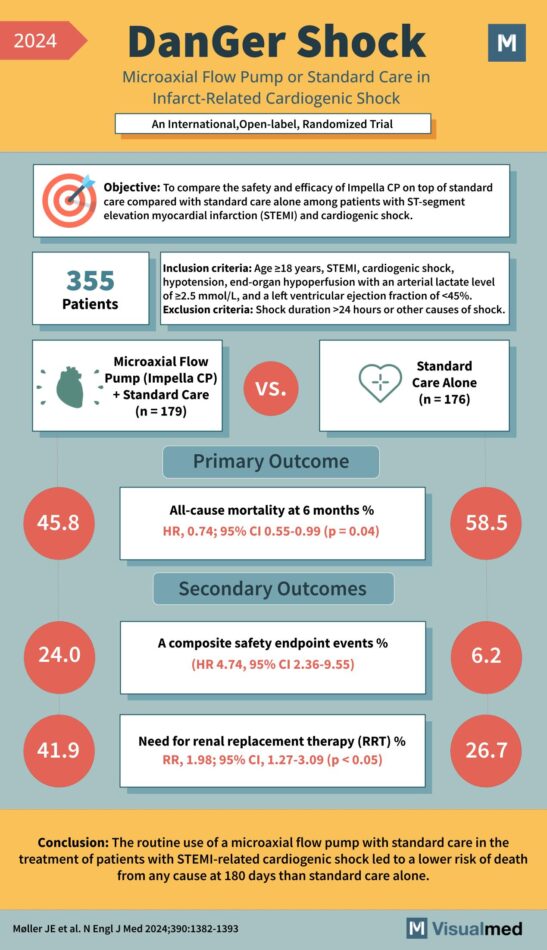
DanGer Shock Trial: Impella in Cardiogenic Shock
“DanGer Shock” study, which is an international, open-label, randomized trial examining the impact of a Microaxial Flow Pump (Impella CP) plus standard care versus standard care alone in infarct-related cardiogenic shock. Objective: To compare the safety and efficacy of Impella … Read More
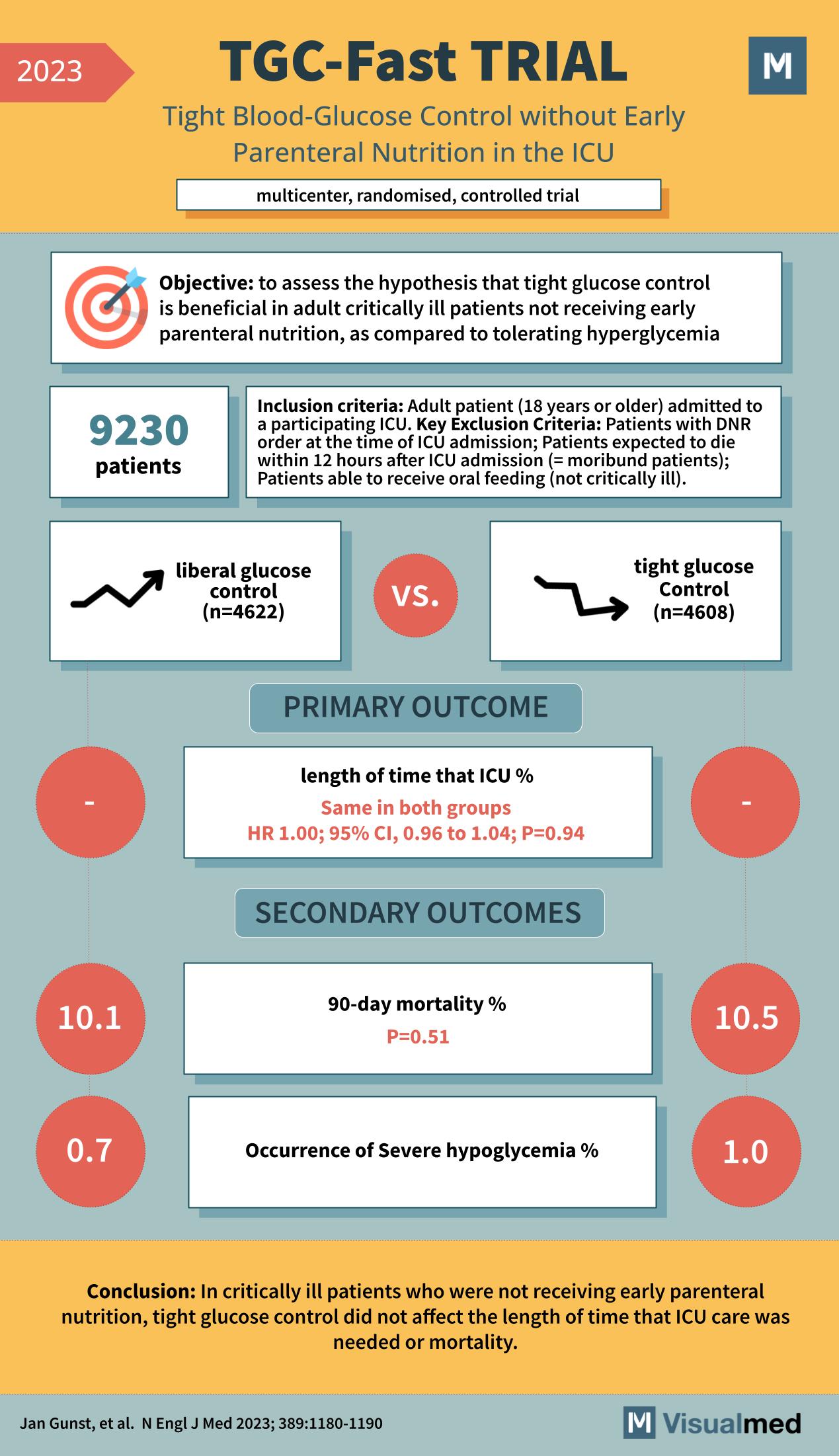
TGC-Fast Trial: Tight Glucose Control in ICU
The TGC-Fast Trial, featured in the New England Journal of Medicine in 2023, was a multicenter, randomized, controlled trial that aimed to assess the benefits of tight glucose control without early parenteral nutrition in the intensive care unit (ICU). The … Read More
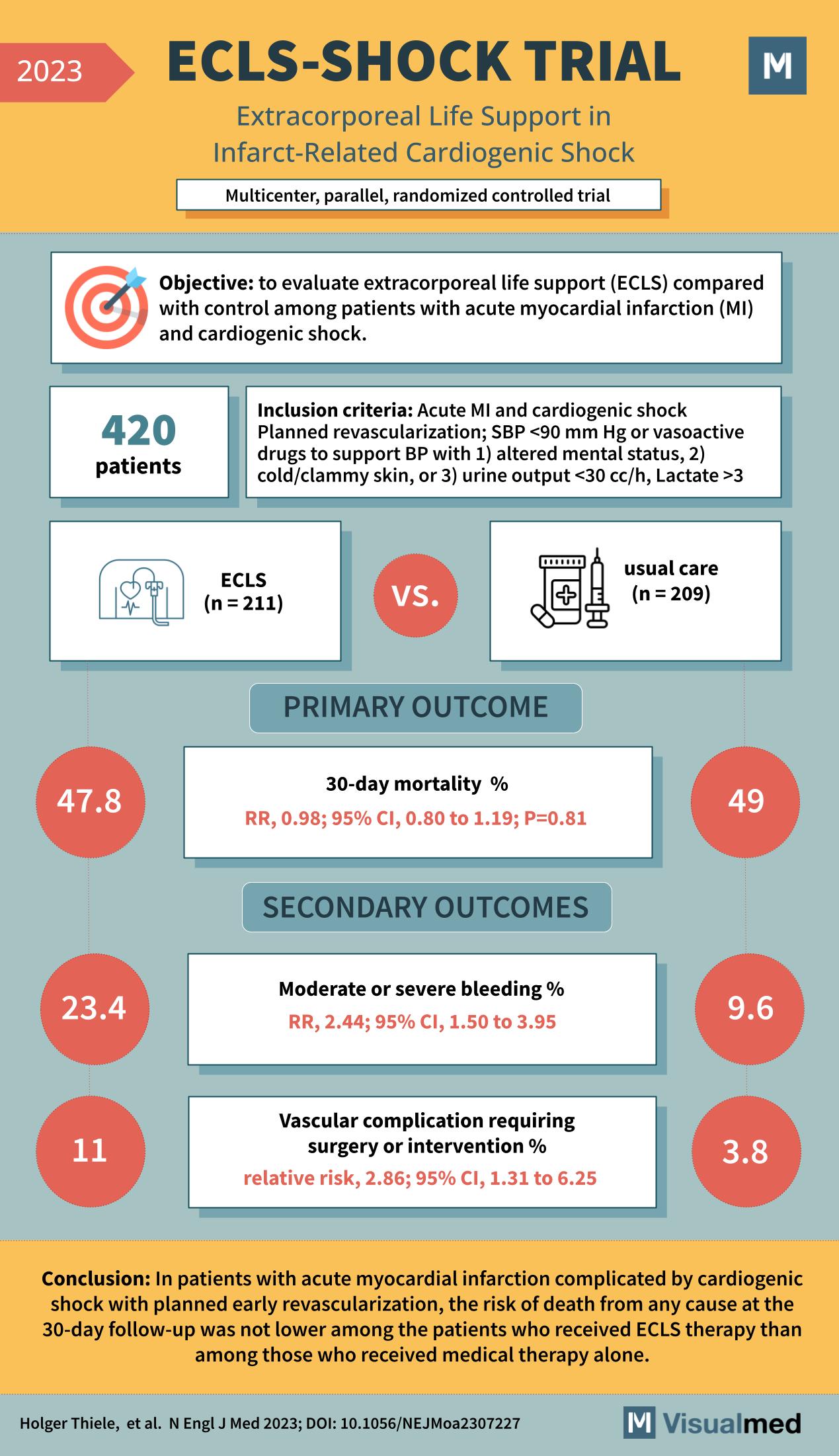
ECLS-SHOCK Trial: Extracorporeal Life Support in Cardiogenic Shock
The ECLS-SHOCK trial, as reported in the New England Journal of Medicine in 2023, was a multicenter, parallel, randomized controlled trial designed to evaluate the efficacy of extracorporeal life support (ECLS) in patients with acute myocardial infarction (MI) complicated by … Read More
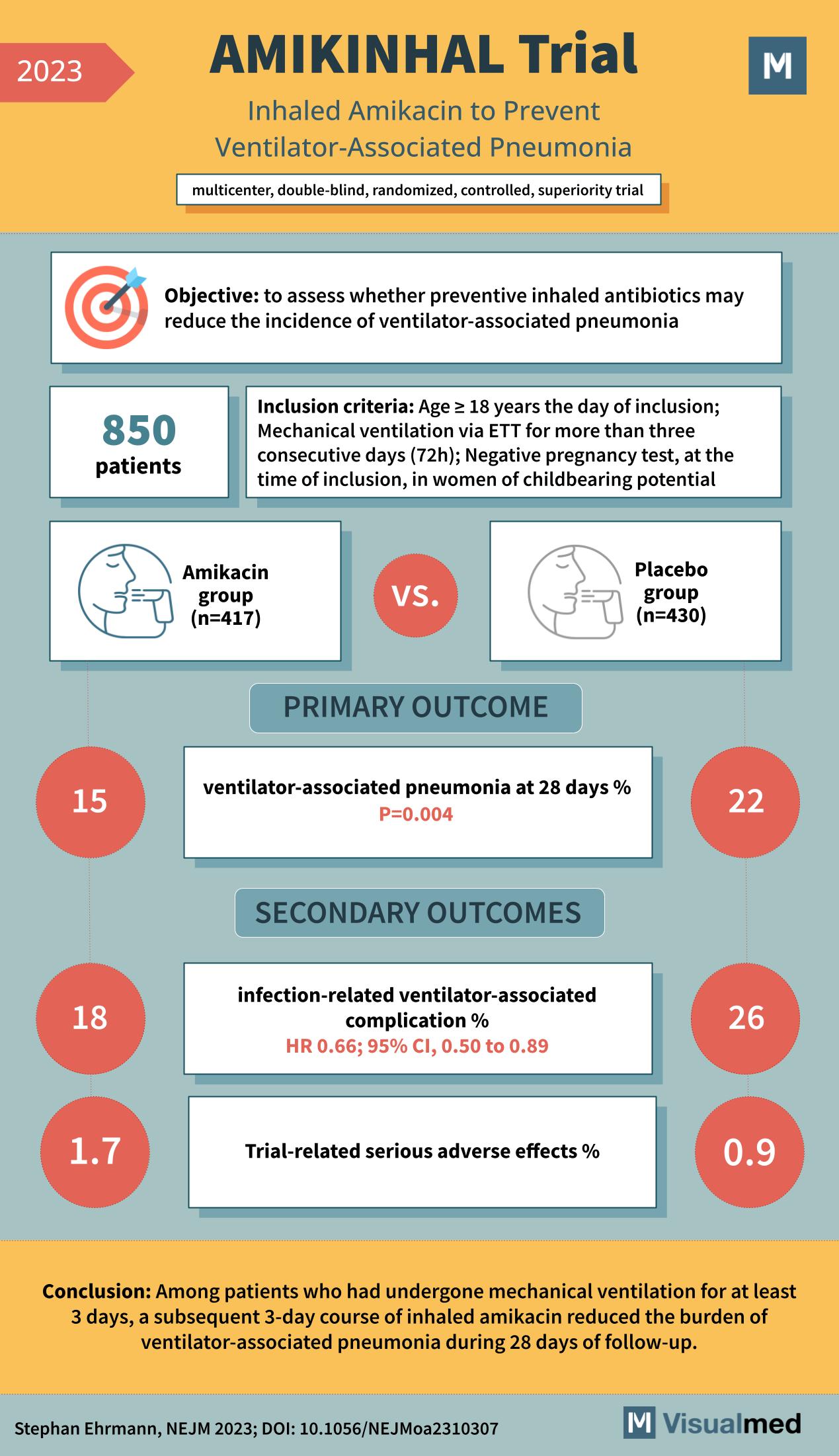
AMIKINHAL Trial: Inhaled Amikacin in Preventing VAP
The medical world is continually seeking innovative methods to combat and prevent life-threatening conditions. One such condition is ventilator-associated pneumonia, a severe complication that affects patients undergoing mechanical ventilation. The recent AMIKINHAL trial, a multicenter, double-blind, randomized, controlled, superiority study, … Read More
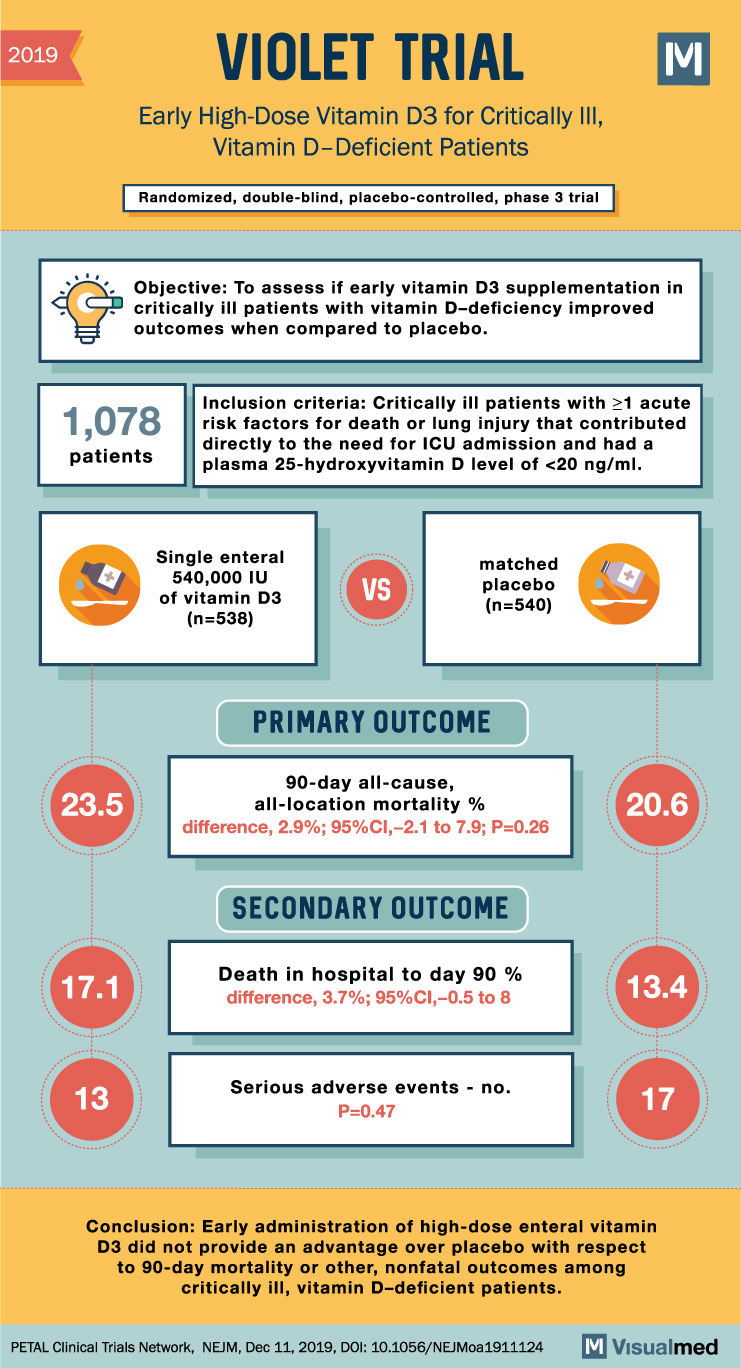
VIOLET Trial: High-Dose Vitamin D3 for Critically Ill
VIOLET Trial Summary The VIOLET trial was a phase 3, randomized, double-blind, placebo-controlled study designed to evaluate the effects of early vitamin D3 supplementation in critically ill patients with vitamin D deficiency who were at high risk for death. Vitamin … Read More
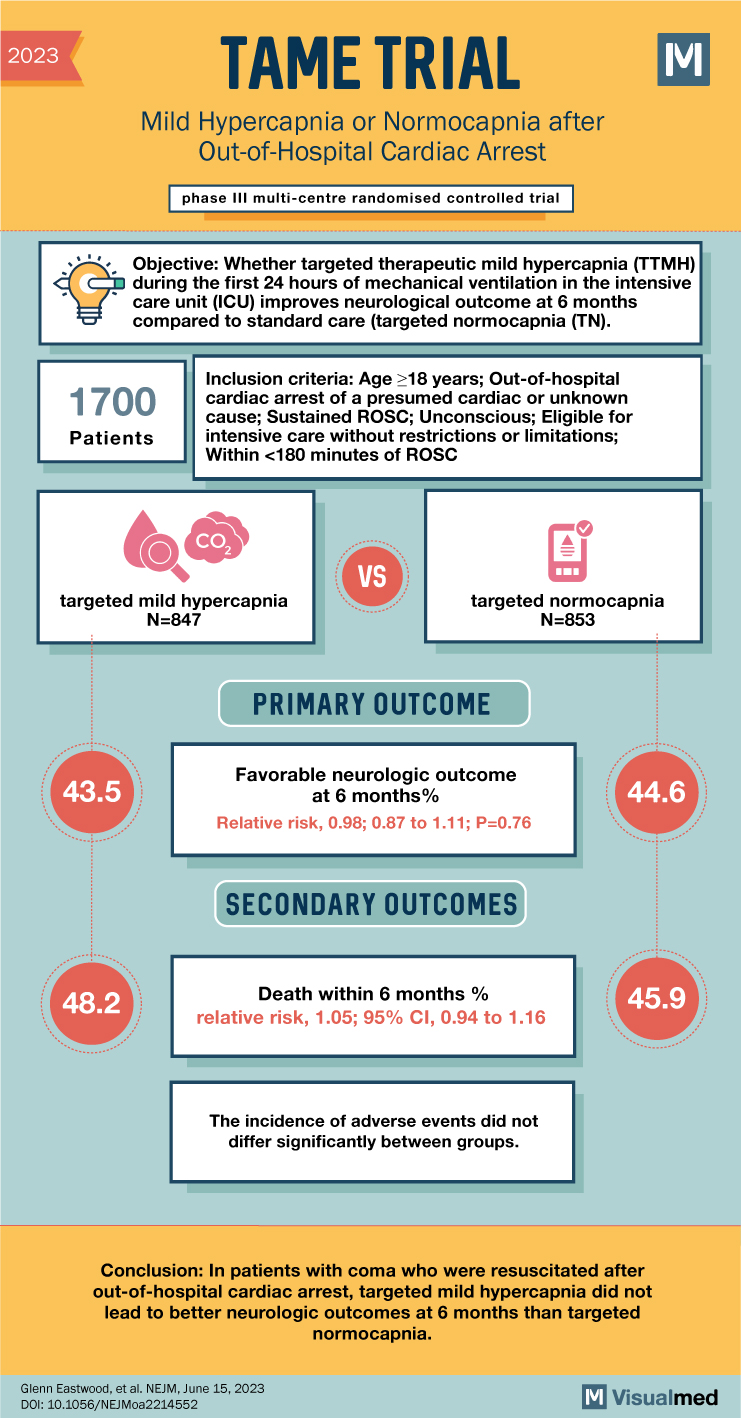
TAME Trial: Mild Hypercapnia after Cardiac Arrest
TAME Trial Summary The TAME trial investigated the impact of mild hypercapnia versus normocapnia on neurologic outcomes in adults with coma who were resuscitated after out-of-hospital cardiac arrest. Current guidelines recommend normocapnia, but mild hypercapnia has been suggested to enhance … Read More
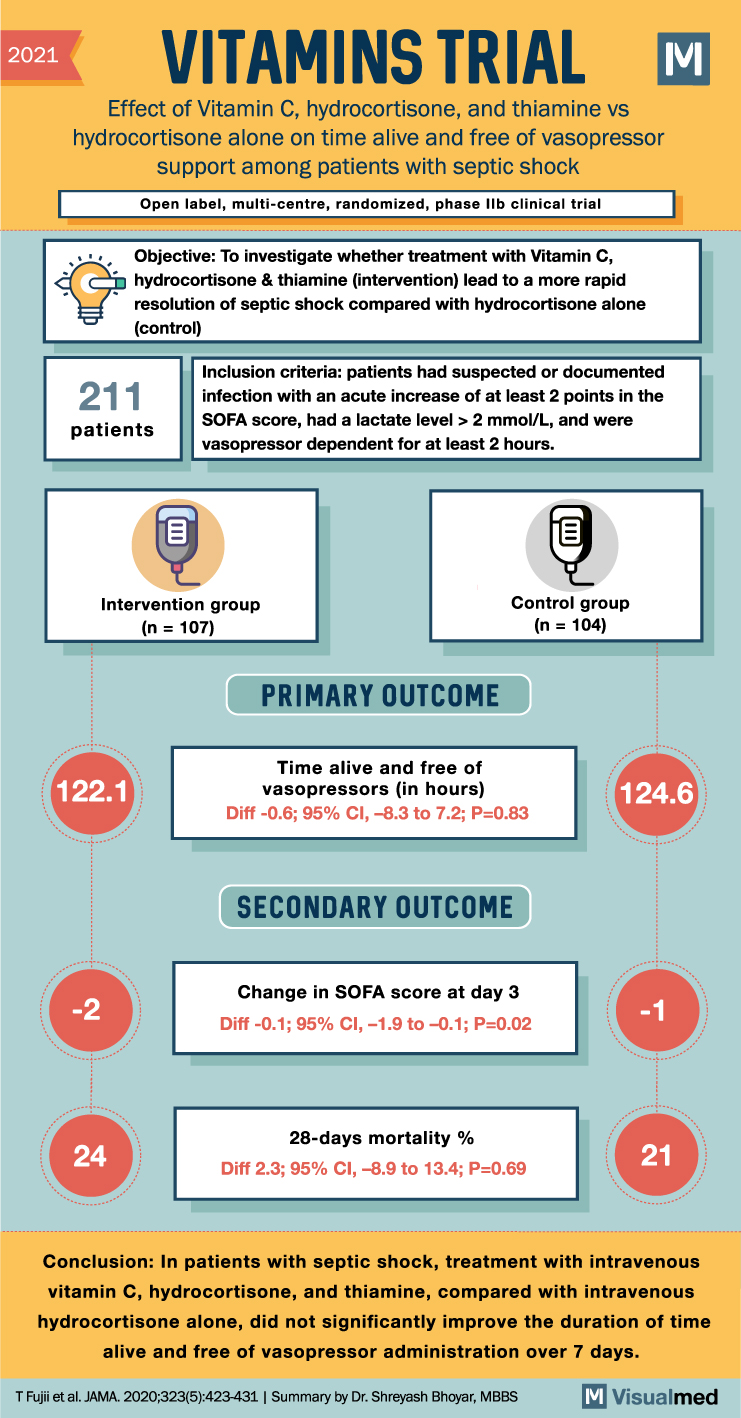
VITAMINS Trial Summary: Vit. C in Septic Shock
VITAMINS Trial Summary The VITAMINS trial aimed to determine whether the combination of vitamin C, hydrocortisone, and thiamine is more effective than hydrocortisone alone in expediting the resolution of septic shock. The trial included 216 patients with septic shock and … Read More
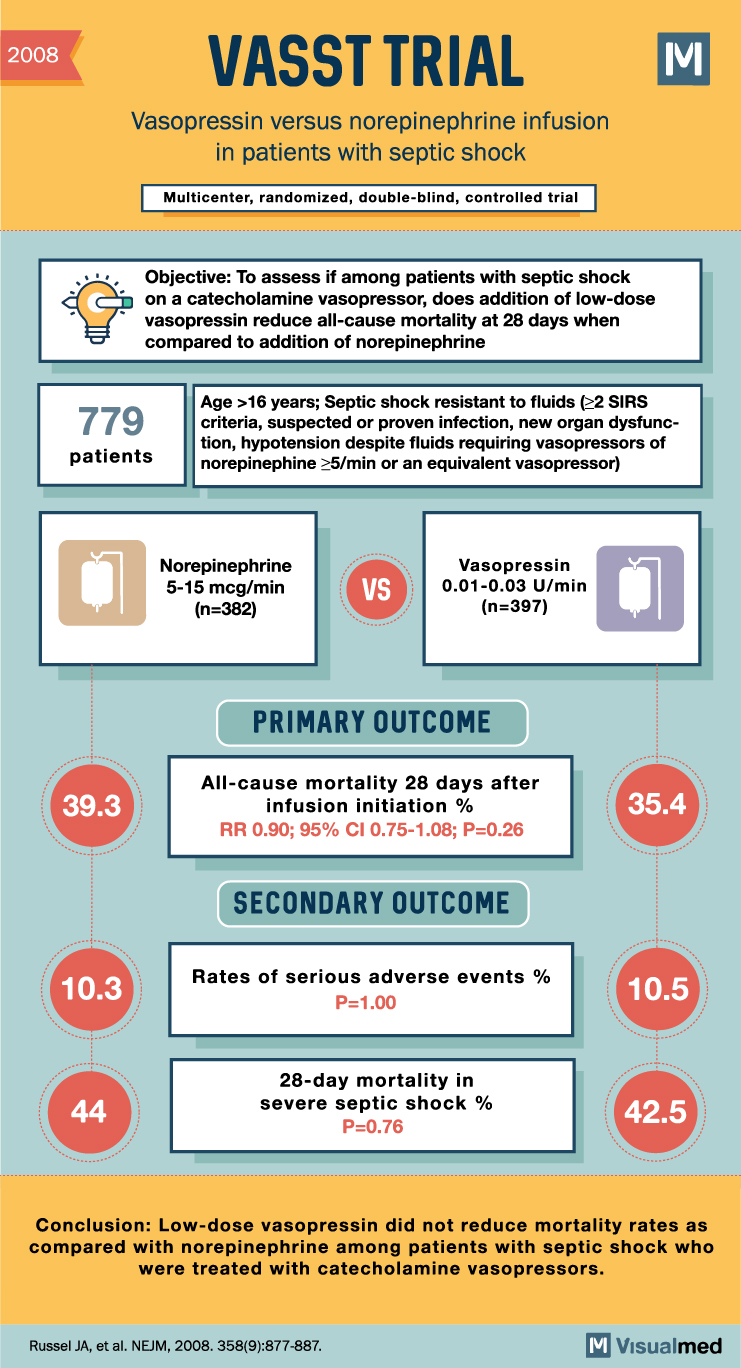
Navigating the Controversy: A Comprehensive Review of Landmark Clinical Trials on Steroids Use in Septic Shock
Explore the pivotal clinical trials that have shaped our understanding of the role of steroids in the management of septic shock, unveiling the complexities and nuances of this controversial topic. Introduction Septic shock is a life-threatening condition characterized by a … Read More
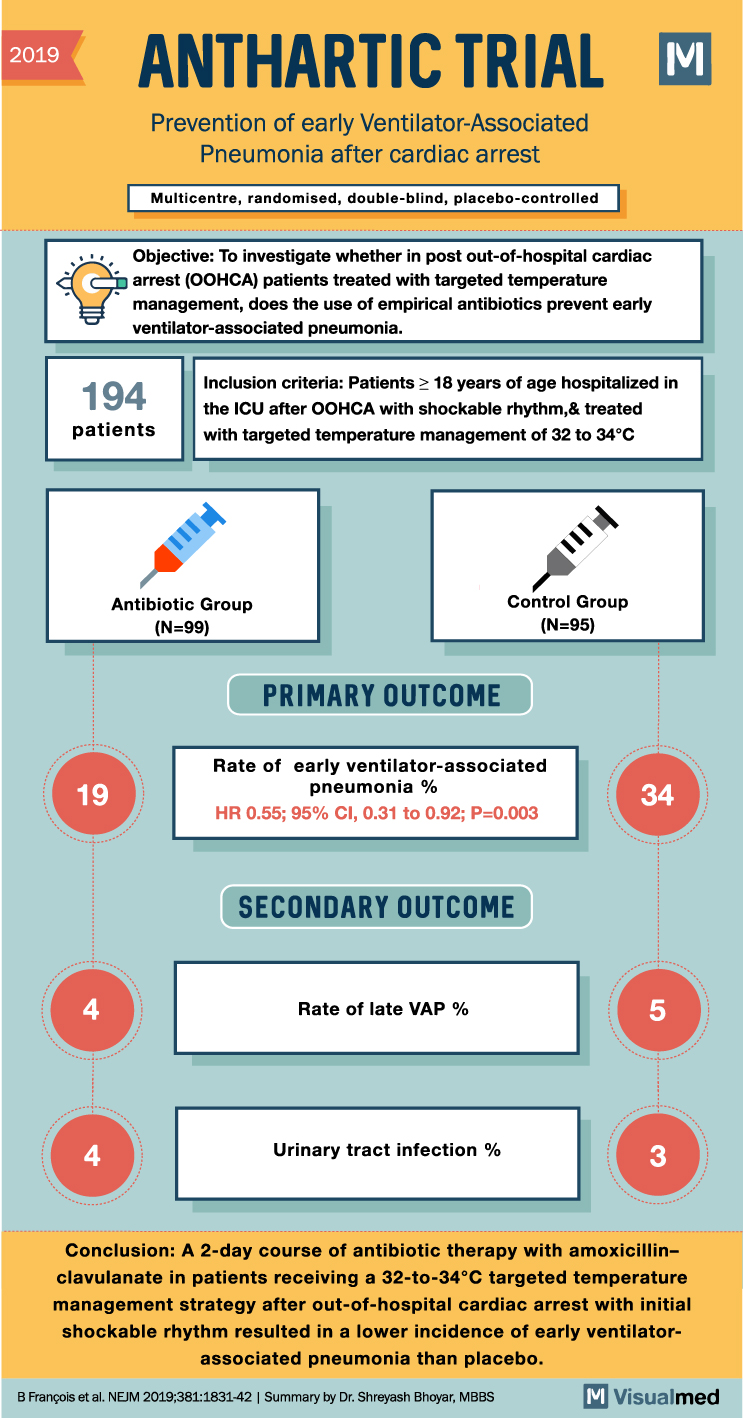
ANTHARTIC Trial: Preventing VAP after CA
2019 ANTHARTIC TRIAL M Prevention of early Ventilator-Associated Pneumonia after cardiac arrest Multicentre, randomised, double-blind, placebo-controlled Objective: To investigate whether in post out-of-hospital cardiac arrest (OOHCA) patients treated with targeted temperature management, does the use of empirical antibiotics prevent early … Read More
