The medical world is continually seeking innovative methods to combat and prevent life-threatening conditions. One such condition is ventilator-associated pneumonia, a severe complication that affects patients undergoing mechanical ventilation. The recent AMIKINHAL trial, a multicenter, double-blind, randomized, controlled, superiority study, presents promising results for the utilization of inhaled amikacin as a preventive measure against this type of pneumonia.
Understanding the AMIKINHAL Trial
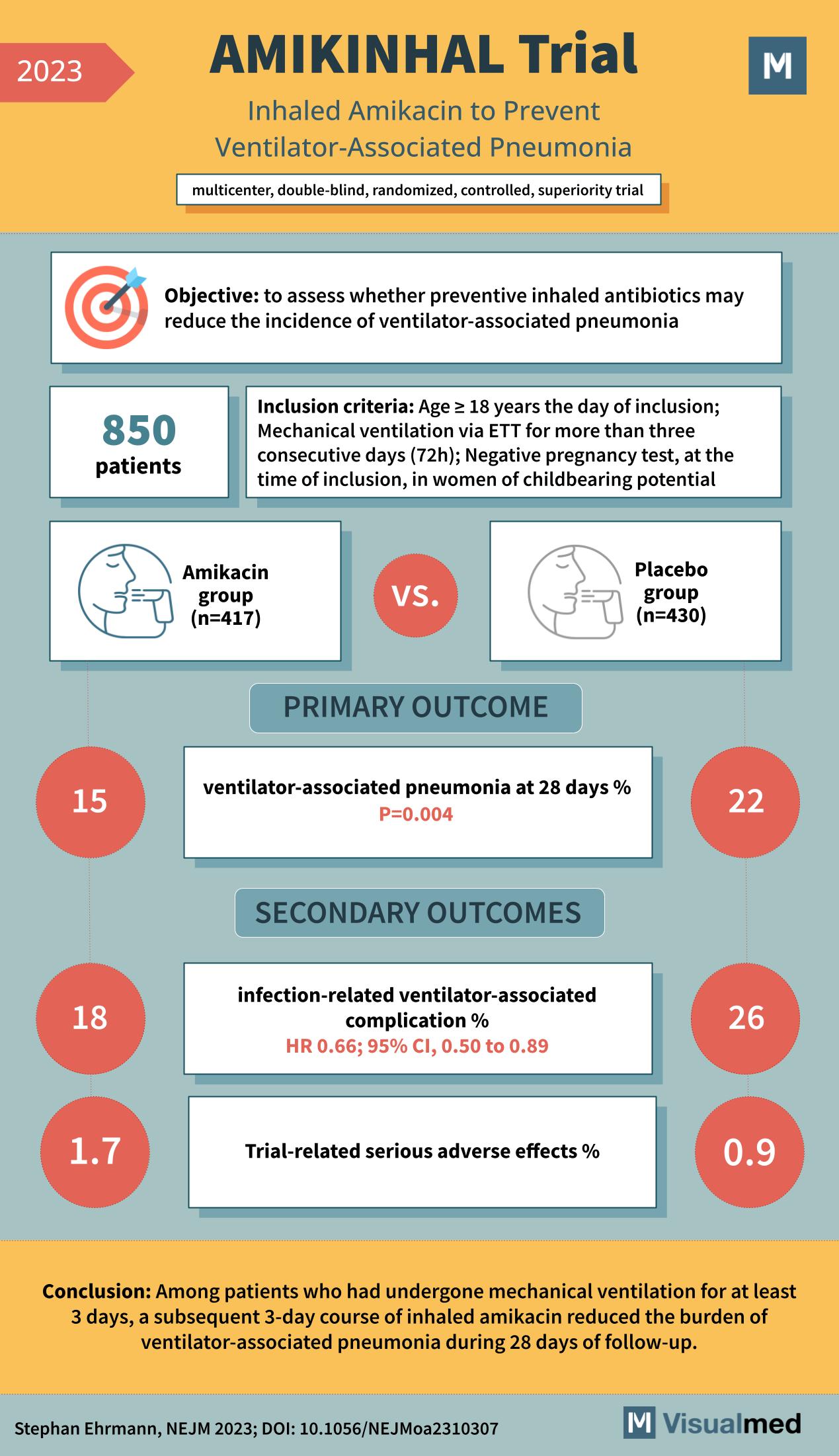
The AMIKINHAL trial was aimed to evaluate whether preventive inhaled antibiotics could reduce the incidence of ventilator-associated pneumonia. The trial was extensive, involving 850 patients, and came with specific inclusion criteria:
- Patients aged 18 years or older on the day of inclusion.
- Those who underwent mechanical ventilation via ETT for more than three consecutive days (72h).
- Women of childbearing potential had to have a negative pregnancy test at the time of inclusion.
Amikacin vs. Placebo: A Comparative Study
The 850 patients were divided into two groups:
- Amikacin Group: Comprising 417 patients, this group was administered with the inhaled form of amikacin.
- Placebo Group: Comprising 430 patients, this group was given a placebo.
The Impressive Outcomes
The primary outcome focused on the incidence of ventilator-associated pneumonia within 28 days of treatment. The results were groundbreaking:
- Amikacin Group: Only 15% of patients developed ventilator-associated pneumonia.
- Placebo Group: A significantly higher 22% of patients developed the condition.
The secondary outcomes, which centered on infection-related ventilator-associated complications and trial-related adverse effects, were equally revealing:
- In the Amikacin group, 18% of patients faced infection-related ventilator-associated complications with a hazard ratio (HR) of 0.66 and a 95% confidence interval (CI) of 0.50 to 0.89. In contrast, 26% of the Placebo group encountered such complications.
- Serious adverse effects related to the trial were lower in both groups: 1.7% in the Amikacin group versus 0.9% in the Placebo group.
Drawing Conclusions
Based on the AMIKINHAL trial results, among patients who had undergone mechanical ventilation for a minimum of three days, a subsequent three-day course of inhaled amikacin notably reduced the burden of ventilator-associated pneumonia during the 28 days of follow-up. This outcome underscores the potential of inhaled amikacin as an efficient preventive measure against this form of pneumonia.
Final Thoughts
Pneumonia, especially when associated with ventilation, can pose severe health risks. The AMIKINHAL trial illuminates the groundbreaking potential of inhaled amikacin as a preventive strategy. As the medical community continues to strive for better patient outcomes, such trials become instrumental in shaping the future of healthcare. The hope is that with continued research and advancements, pneumonia’s burden can be further reduced, ensuring safer mechanical ventilation procedures and better patient health.