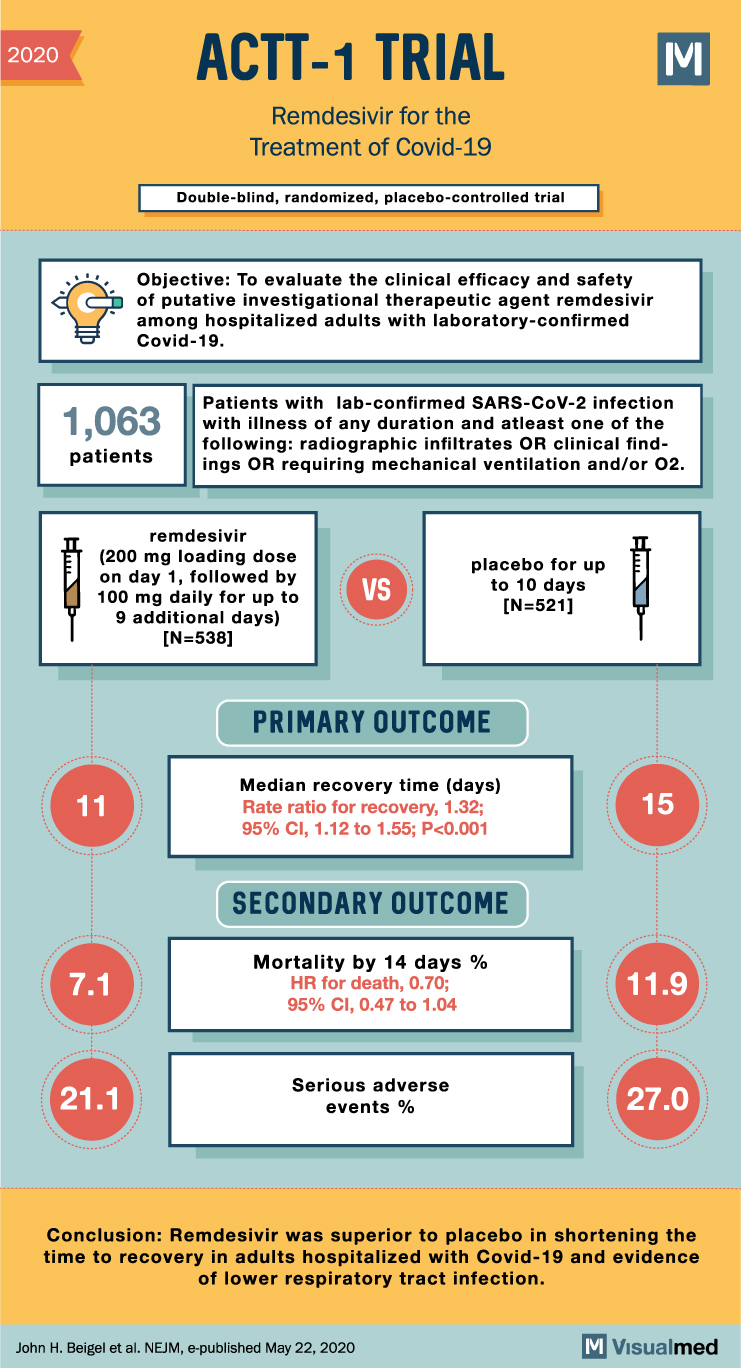
2020 ACTT-1 TRIAL Remdesivir for the Treatment of Covid-19 Double-blind, randomized, placebo-controlled trial Objective: To evaluate the clinical efficacy and safety of putative investigational therapeutic agent remdesivir among hospitalized adults with laboratory-confirmed Covid-19. M Patients with lab-confirmed SARS-CoV-2 infection 1,063 with illness of any duration and atleast one of the patients following: radiographic infiltrates OR clinical find- ings OR requiring mechanical ventilation and/or 02. remdesivir (200 mg loading dose on day 1, followed by 100 mg daily for up to 9 additional days) [N=538] VS placebo for up to 10 days [N=521] 11 PRIMARY OUTCOME Median recovery time (days) Rate ratio for recovery, 1.32; 95% CI, 1.12 to 1.55; P<0.001 7.1 SECONDARY OUTCOME Mortality by 14 days % HR for death, 0.70; 95% CI, 0.47 to 1.04 15 11.9 21.1 Serious adverse events % 27.0 Conclusion: Remdesivir was superior to placebo in shortening the time to recovery in adults hospitalized with Covid-19 and evidence of lower respiratory tract infection. John H. Beigel et al. NEJM, e-published May 22, 2020