WRAP-IT Trial Summary
Infections following the placement of cardiac implantable electronic devices (CIEDs) can lead to significant morbidity and mortality. Although preoperative antibiotics are a standard prophylactic strategy, evidence for other preventive approaches is limited. The WRAP-IT trial sought to examine the efficacy and safety of an absorbable, antibiotic-eluting envelope in reducing CIED-associated infections.
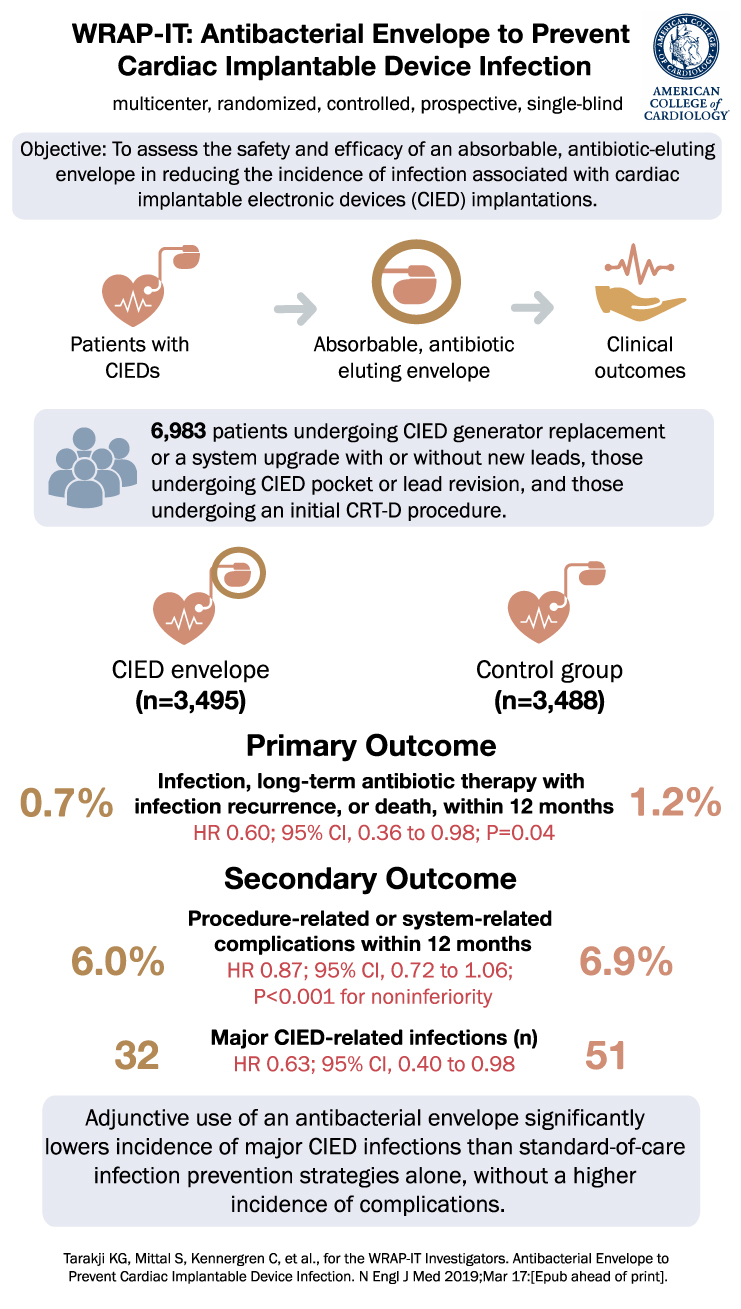
The WRAP-IT trial was a randomized, controlled clinical study involving patients undergoing CIED pocket revision, generator replacement, system upgrade, or initial implantation of a cardiac resynchronization therapy defibrillator. Patients were randomly assigned in a 1:1 ratio to either receive the antibiotic-eluting envelope or not. Standard-of-care strategies for infection prevention were utilized in all patients. The primary endpoint was the occurrence of infection necessitating system extraction or revision, long-term antibiotic therapy with infection recurrence, or death, within 12 months after the CIED implantation procedure. The secondary endpoint was procedure-related or system-related complications within 12 months.
A total of 6983 patients were randomized, with 3495 assigned to the envelope group and 3488 to the control group. The primary endpoint occurred in 25 patients in the envelope group and 42 patients in the control group. The 12-month Kaplan-Meier estimated event rate was 0.7% in the envelope group and 1.2% in the control group (hazard ratio, 0.60; 95% confidence interval [CI], 0.36 to 0.98; P=0.04). The safety endpoint occurred in 201 patients in the envelope group and 236 patients in the control group, with a 12-month Kaplan-Meier estimated event rate of 6.0% and 6.9% respectively (hazard ratio, 0.87; 95% CI, 0.72 to 1.06; P<0.001 for noninferiority).
The mean duration of follow-up was 20.7±8.5 months. Major CIED-related infections throughout the entire follow-up period occurred in 32 patients in the envelope group and 51 patients in the control group (hazard ratio, 0.63; 95% CI, 0.40 to 0.98).
In conclusion, the findings from the WRAP-IT trial demonstrate that the adjunctive use of an antibacterial envelope significantly reduces the incidence of major CIED infections compared to standard-of-care infection-prevention strategies alone. Furthermore, this approach does not increase the incidence of complications. These results offer valuable insights into new strategies for preventing CIED-associated infections, and they signal a potential advancement in patient care in the field of cardiac device implantation.