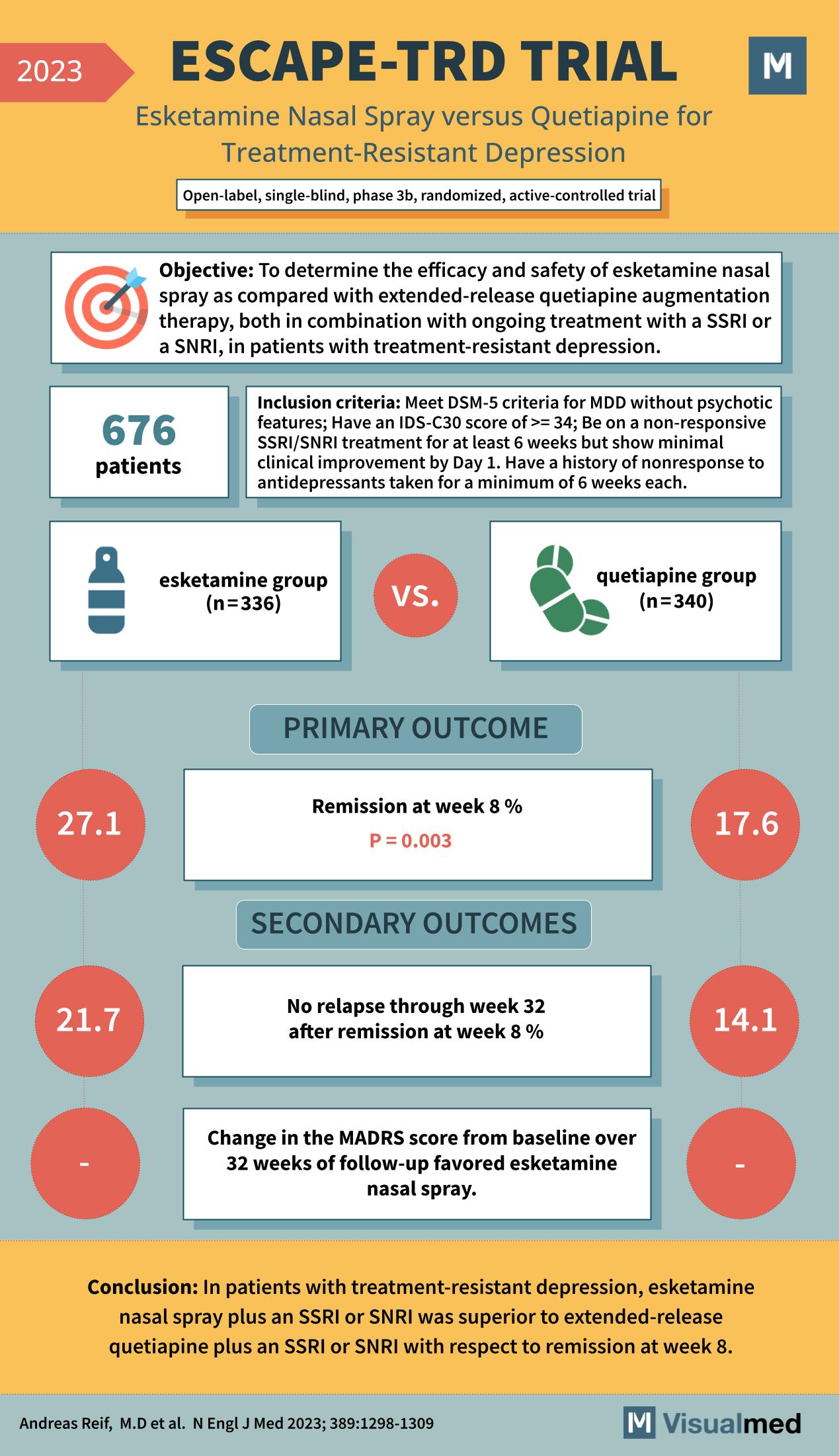
The ESCAPE-TRD trial, as reported in the New England Journal of Medicine in 2023, stands as a significant landmark in the ongoing quest to improve treatments for treatment-resistant depression (TRD). This trial assessed the efficacy and safety of esketamine nasal spray in comparison with extended-release quetiapine, each in combination with an ongoing selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) therapy.
With 676 patients participating, the open-label, single-blind, phase 3b, randomized, active-controlled ESCAPE-TRD trial included individuals who met the DSM-5 criteria for Major Depressive Disorder (MDD) without psychotic features and had an Inventory of Depressive Symptomatology Clinician-rated 30-item (IDS-C30) score of at least 34. These patients had not responded adequately to SSRI/SNRI treatments and showed minimal clinical improvement despite ongoing treatment.
The primary outcome was the percentage of patients achieving remission at week 8, which was significantly higher in the esketamine group (27.1%) compared to the quetiapine group (17.6%), with a p-value of 0.003. This indicated that the esketamine nasal spray, when used as an adjunctive treatment, was more effective at achieving remission in TRD patients than quetiapine.
Secondary outcomes included the rate of relapse through week 32 after achieving remission at week 8. The esketamine group had a lower relapse rate of 21.7%, compared to 14.1% in the quetiapine group, suggesting a more sustained response with esketamine. Additionally, changes in the Montgomery-Åsberg Depression Rating Scale (MADRS) scores from baseline over 32 weeks of follow-up favored the esketamine nasal spray, although specific numbers were not provided in the visual summary.
The conclusion drawn from the ESCAPE-TRD trial is particularly encouraging for those grappling with TRD. Esketamine nasal spray plus an SSRI or SNRI was superior to extended-release quetiapine plus an SSRI or SNRI in achieving remission at week 8. This suggests that esketamine could be a valuable addition to the treatment regimen for patients with TRD who have not responded to standard antidepressant therapies.
This trial contributes valuable data to the field of psychiatry, offering a potential new treatment pathway for one of the most challenging conditions to treat effectively. It underscores the importance of considering novel pharmacological approaches when conventional treatments fail to yield sufficient clinical benefits.
The ESCAPE-TRD trial’s findings have profound implications for clinical practice, potentially changing the way clinicians approach TRD management. With a significant portion of patients with MDD not responding to first-line treatments, esketamine presents an alternative that could benefit many who are in need of more effective treatment options.
As mental health treatments continue to evolve, the ESCAPE-TRD trial is a reminder of the necessity for ongoing research and innovation to provide relief for patients with TRD, a condition that significantly impacts quality of life and carries a substantial burden for individuals and healthcare systems alike.