ARTESIA Trial Summary
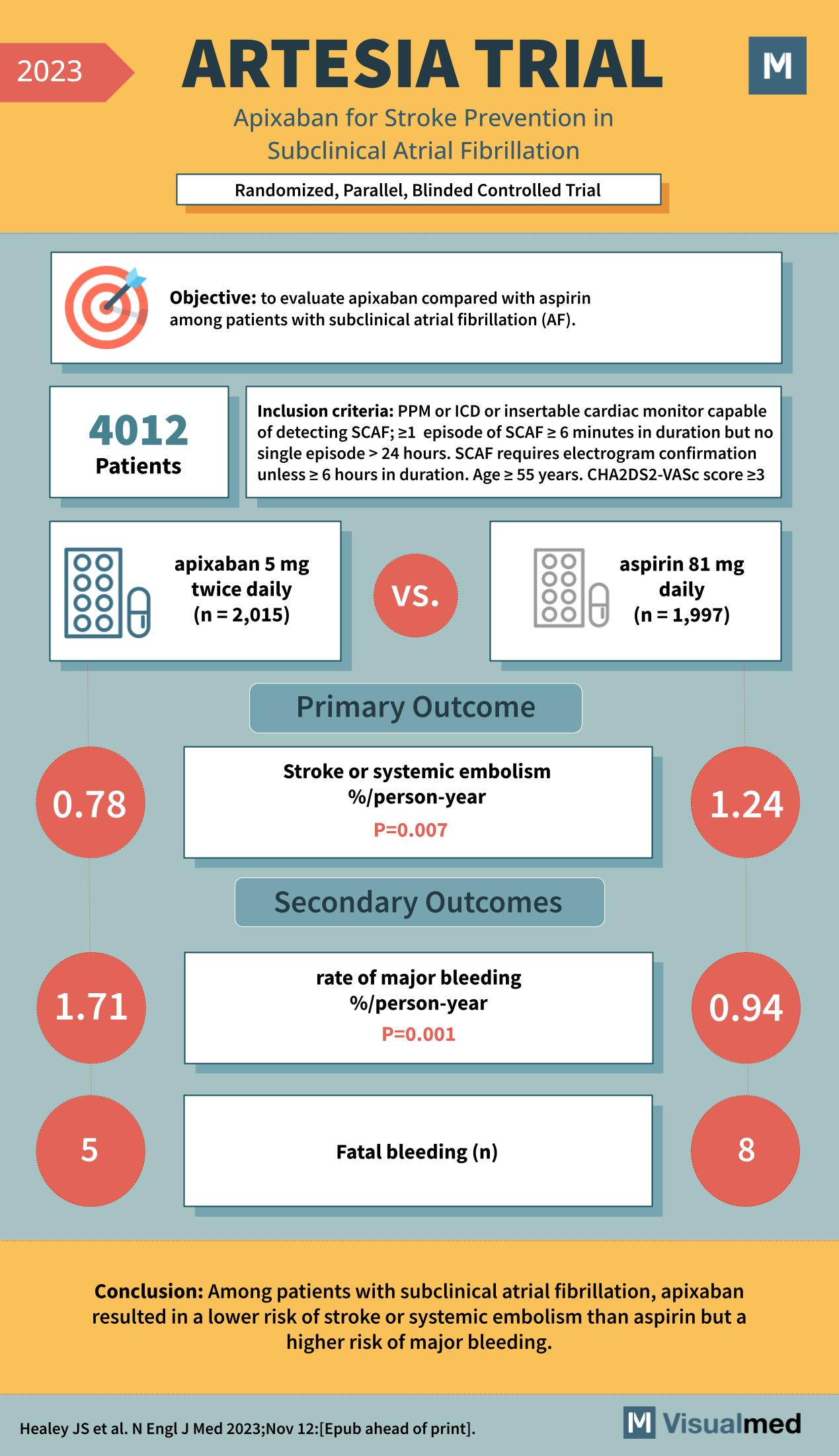
The ARTESIA trial, which stands for “Apixaban for Stroke Prevention in Subclinical Atrial Fibrillation,” was a landmark study aimed at comparing the efficacy and safety of apixaban versus aspirin in patients with subclinical atrial fibrillation (AF). The clinical trial was randomized, parallel, and blinded, ensuring that the results were unbiased and reliable.
The objective of the ARTESIA trial was to evaluate whether apixaban, an anticoagulant, was superior to aspirin, an antiplatelet agent, in preventing strokes or systemic embolism in patients with subclinical atrial fibrillation. Subclinical AF is a condition where patients have episodes of AF that are not symptomatic. These episodes are usually detected incidentally during routine examinations or monitoring.
A total of 4,012 patients were enrolled in the trial. They were split into two groups, with 2,015 patients receiving 5 mg of apixaban twice daily and 1,997 patients receiving 81 mg of aspirin daily. The inclusion criteria for the study were quite stringent, including patients with a pacemaker (PPM) or implantable cardioverter-defibrillator (ICD) or those with an insertable cardiac monitor capable of detecting subclinical AF episodes. These episodes had to be at least 6 minutes in duration but no single episode longer than 24 hours. Additionally, an electrogram confirmation was required unless the episode was less than 6 hours in duration. Eligible patients were aged 55 years or above and had a CHA2DS2-VASc score of 3 or higher, indicating a moderate to high risk of stroke.
The primary outcome measure was the incidence of stroke or systemic embolism, reported as a percentage per person-year. The secondary outcomes included the rate of major bleeding, also reported as a percentage per person-year, and the number of fatal bleeding events.
The results of the ARTESIA trial were significant. The incidence of stroke or systemic embolism in the apixaban group was 0.78% per person-year, compared to 1.24% per person-year in the aspirin group. This difference was statistically significant, with a p-value of 0.007, indicating that apixaban was more effective than aspirin in preventing stroke or systemic embolism. However, the rate of major bleeding was higher in the apixaban group, at 1.71% per person-year versus 0.94% per person-year in the aspirin group, with a p-value of 0.001. There were also more fatal bleeding events in the apixaban group, with 5 cases compared to 8 cases in the aspirin group.
The conclusion drawn from the ARTESIA trial was that among patients with subclinical atrial fibrillation, apixaban resulted in a lower risk of stroke or systemic embolism than aspirin but a higher risk of major bleeding. This finding is crucial as it suggests that while apixaban is more effective at preventing one of the most feared complications of AF, it comes with an increased risk of serious bleeding.
The ARTESIA trial’s findings have significant implications for the management of patients with subclinical AF. Clinicians must weigh the benefits of stroke prevention against the risks of major bleeding when deciding on the best therapeutic approach for their patients. The trial highlights the importance of individualized patient care and may influence future guidelines on the management of subclinical AF.
For healthcare professionals and patients alike, the ARTESIA trial offers critical insights into the risks and benefits of anticoagulation therapy in a patient population that has been underrepresented in clinical research. As with any medical intervention, the choice of anticoagulant therapy should be tailored to the individual patient, taking into account their specific risk factors for stroke and bleeding.