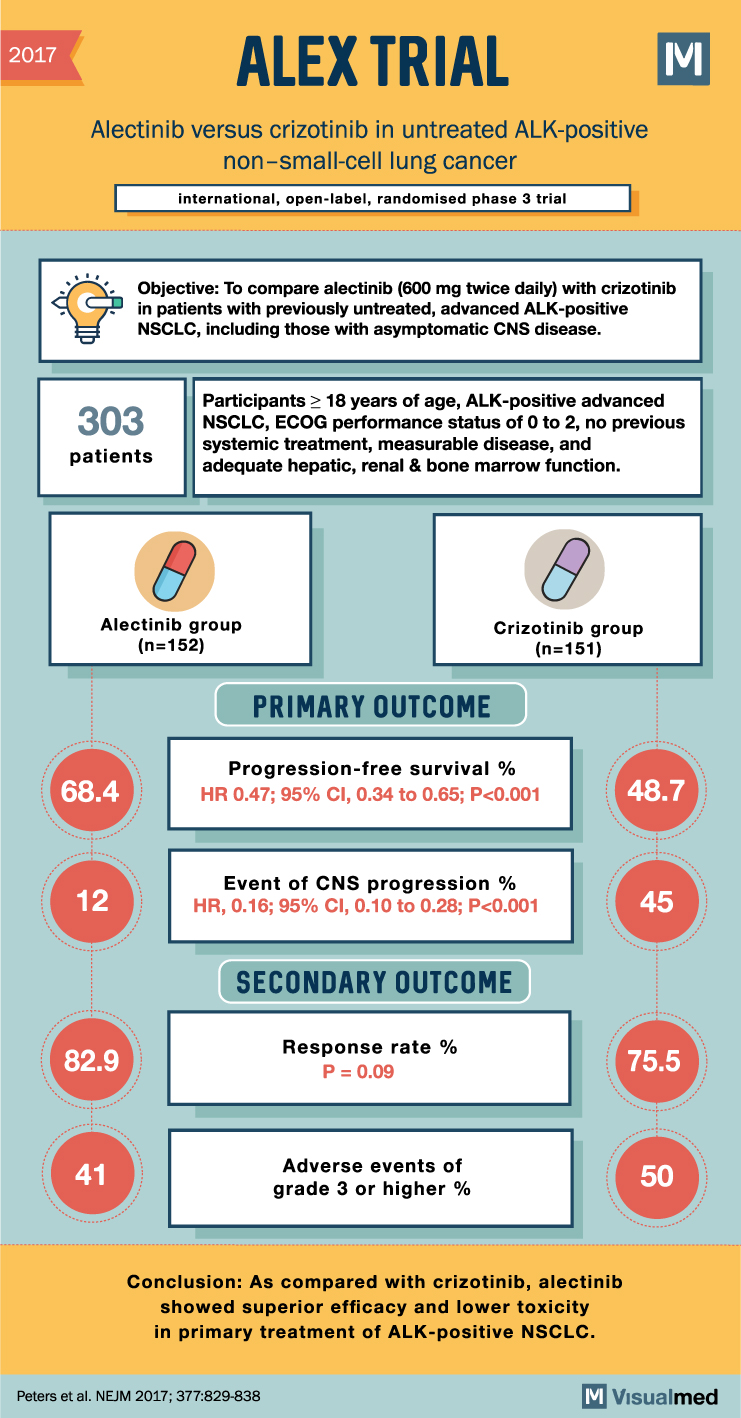
2017 ALEX TRIAL Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer international, open-label, randomised phase 3 trial Σ Objective: To compare alectinib (600 mg twice daily) with crizotinib in patients with previously untreated, advanced ALK-positive NSCLC, including those with asymptomatic CNS disease. 303 patients Participants > 18 years of age, ALK-positive advanced NSCLC, ECOG performance status of 0 to 2, no previous systemic treatment, measurable disease, and adequate hepatic, renal & bone marrow function. Alectinib group (n=152) PRIMARY OUTCOME Crizotinib group (n=151) Progression-free survival % 68.4 HR 0.47; 95% CI, 0.34 to 0.65; P<0.001 48.7 12 Event of CNS progression % HR, 0.16; 95% CI, 0.10 to 0.28; P<0.001 45 SECONDARY OUTCOME 82.9 Response rate % P = 0.09 75.5 41 Adverse events of grade 3 or higher % 50 Conclusion: As compared with crizotinib, alectinib showed superior efficacy and lower toxicity in primary treatment of ALK-positive NSCLC. Peters et al. NEJM 2017; 377:829-838