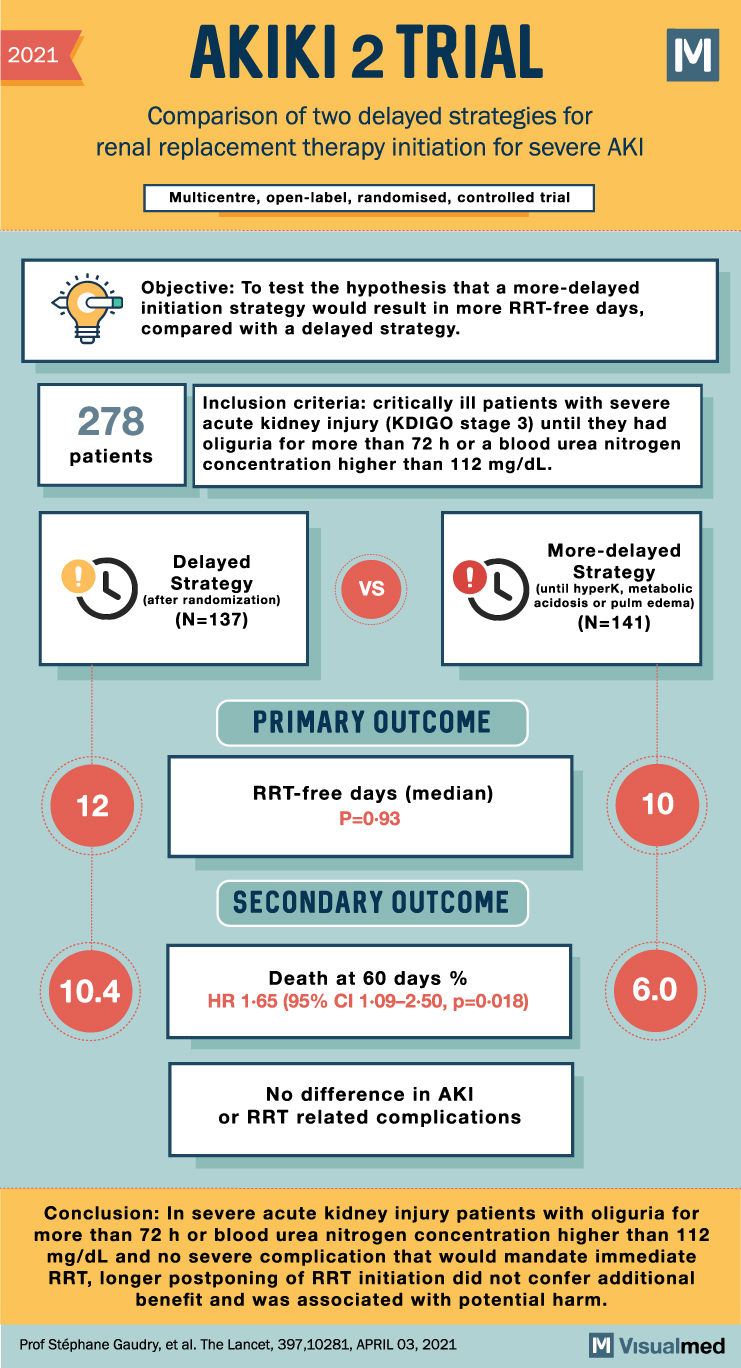
2021 AKIKI 2 TRIAL Comparison of two delayed strategies for renal replacement therapy initiation for severe AKI Multicentre, open-label, randomised, controlled trial Objective: To test the hypothesis that a more-delayed initiation strategy would result in more RRT-free days, compared with a delayed strategy. Σ 278 patients Inclusion criteria: critically ill patients with severe acute kidney injury (KDIGO stage 3) until they had oliguria for more than 72 h or a blood urea nitrogen concentration higher than 112 mg/dL. D Delayed Strategy (after randomization) (N=137) VS PRIMARY OUTCOME More-delayed Strategy (until hyperk, metabolic acidosis or pulm edema) (N=141) 12 RRT-free days (median) P=0.93 10 SECONDARY OUTCOME Death at 60 days % 10.4 6.0 HR 1-65 (95% CI 1-09-2-50, p=0.018) No difference in AKI or RRT related complications Conclusion: In severe acute kidney injury patients with oliguria for more than 72 h or blood urea nitrogen concentration higher than 112 mg/dL and no severe complication that would mandate immediate RRT, longer postponing of RRT initiation did not confer additional benefit and was associated with potential harm. Prof Stéphane Gaudry, et al. The Lancet, 397,10281, APRIL 03, 2021