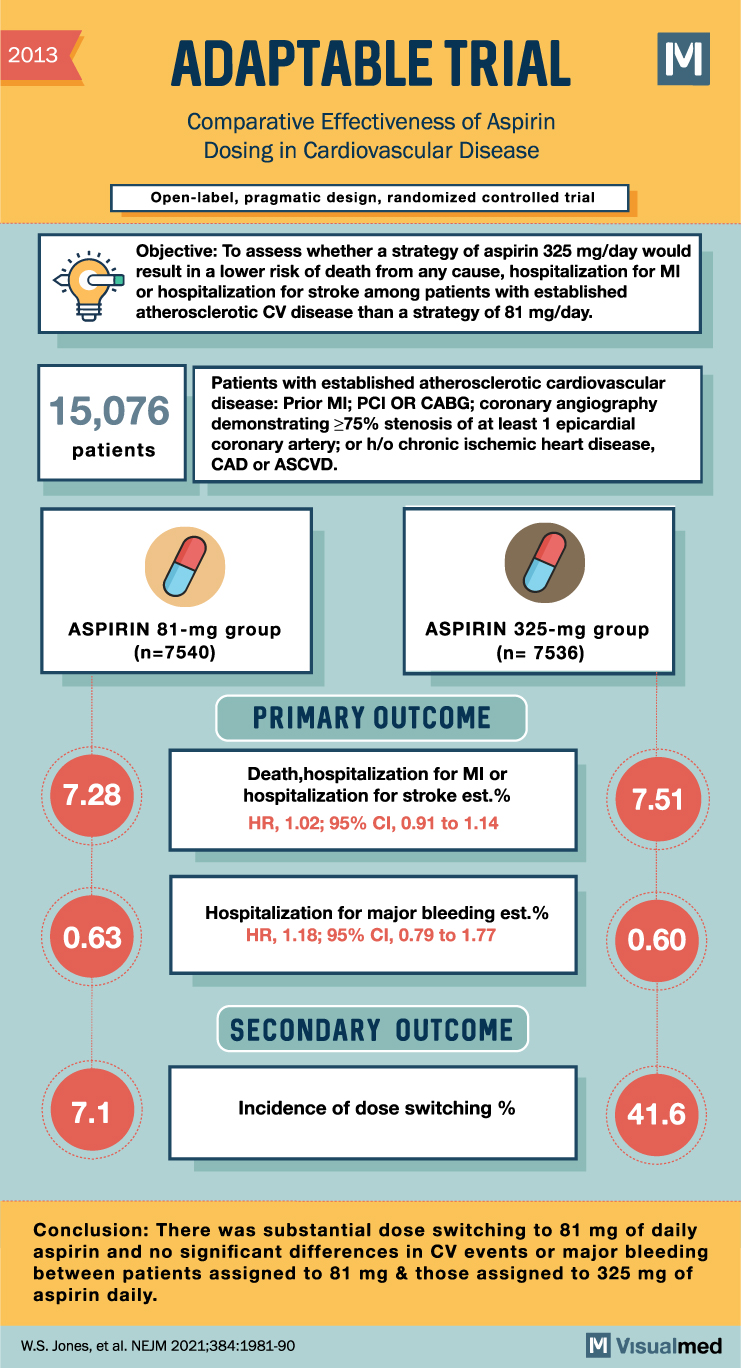
2013 ADAPTABLE TRIAL Comparative Effectiveness of Aspirin Dosing in Cardiovascular Disease Open-label, pragmatic design, randomized controlled trial M Objective: To assess whether a strategy of aspirin 325 mg/day would result in a lower risk of death from any cause, hospitalization for MI or hospitalization for stroke among patients with established atherosclerotic CV disease than a strategy of 81 mg/day. 15,076 patients Patients with established atherosclerotic cardiovascular disease: Prior MI; PCI OR CABG; coronary angiography demonstrating ≥75% stenosis of at least 1 epicardial coronary artery; or h/o chronic ischemic heart disease, CAD, or ASCVD. O ASPIRIN 81-mg group (n=7540) 7.28 ASPIRIN 325-mg group (n= 7536) PRIMARY OUTCOME Death, hospitalization for MI or hospitalization for stroke est.% HR, 1.02; 95% CI, 0.91 to 1.14 7.51 0.63 Hospitalization for major bleeding est.% HR, 1.18; 95% CI, 0.79 to 1.77 0.60 SECONDARY OUTCOME 7.1 Incidence of dose switching % 41.6 Conclusion: There was substantial dose switching to 81 mg of daily aspirin and no significant differences in CV events or major bleeding between patients assigned to 81 mg & those assigned to 325 mg of aspirin daily. W.S. Jones, et al. NEJM 2021;384:1981-90