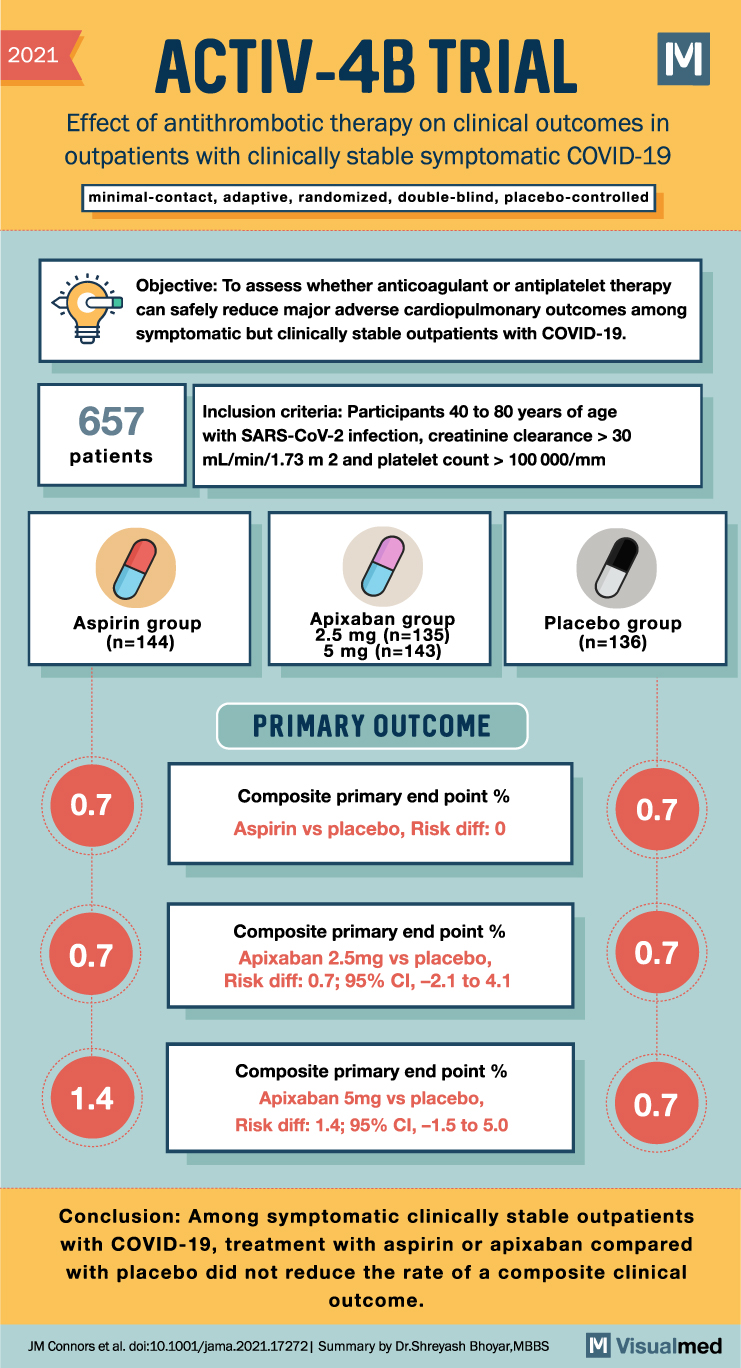
2021 ACTIV-4B TRIAL Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19 minimal-contact, adaptive, randomized, double-blind, placebo-controlled Objective: To assess whether anticoagulant or antiplatelet therapy can safely reduce major adverse cardiopulmonary outcomes among symptomatic but clinically stable outpatients with COVID-19. 657 patients Inclusion criteria: Participants 40 to 80 years of age with SARS-CoV-2 infection, creatinine clearance > 30 mL/min/1.73 m 2 and platelet count > 100 000/mm CD Aspirin group (n=144) Apixaban group 2.5 mg (n=135) 5 mg (n=143) Placebo group (n=136) PRIMARY OUTCOME 0.7 Composite primary end point% 0.7 Aspirin vs placebo, Risk diff: 0 0.7 Composite primary end point% Apixaban 2.5mg vs placebo, Risk diff: 0.7; 95% CI, -2.1 to 4.1 0.7 Composite primary end point % 1.4 Apixaban 5mg vs placebo, 0.7 Risk diff: 1.4; 95% CI, -1.5 to 5.0 Conclusion: Among symptomatic clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome. JM Connors et al. doi:10.1001/jama.2021.17272