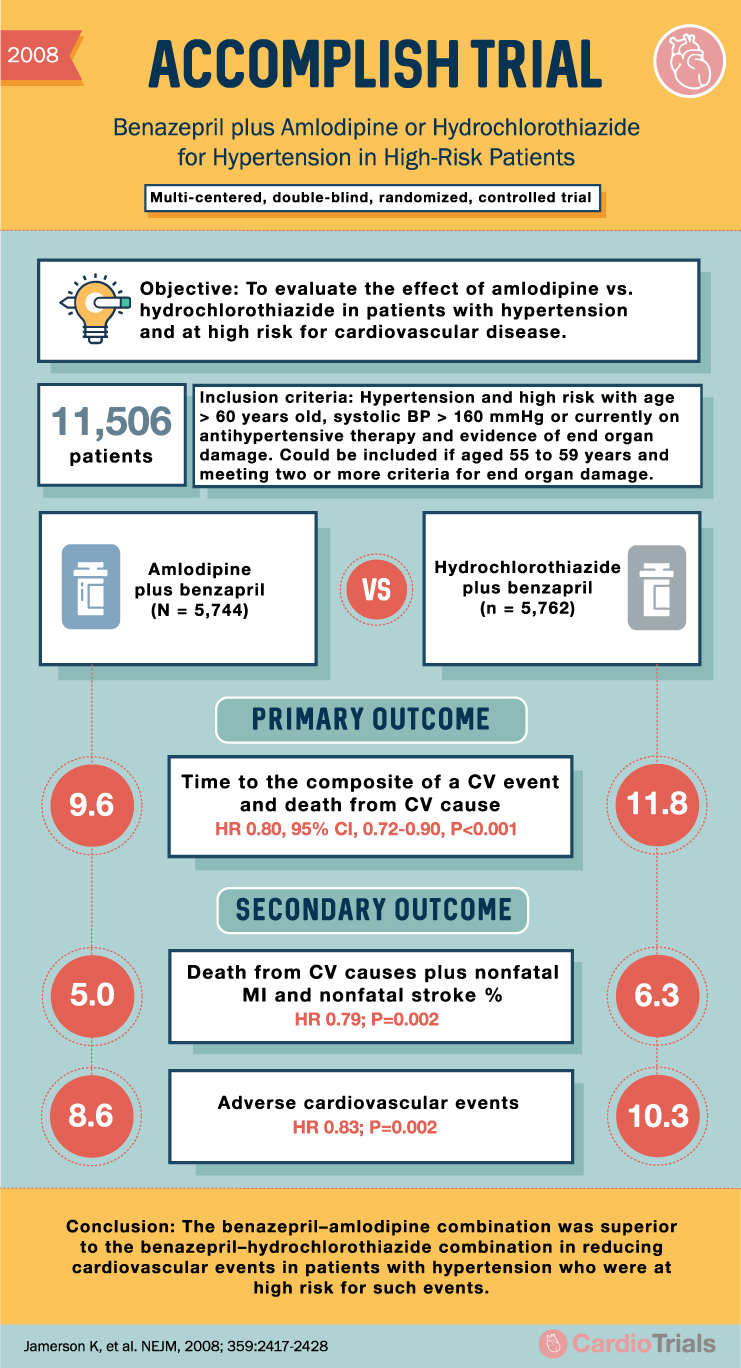
2008 ACCOMPLISH TRIAL Benazepril plus Amlodipine or Hydrochlorothiazide for Hypertension in High-Risk Patients Multi-centered, double-blind, randomized, controlled trial Objective: To evaluate the effect of amlodipine vs. hydrochlorothiazide in patients with hypertension and at high risk for cardiovascular disease. E 11,506 Inclusion criteria: Hypertension and high risk with age > 60 years old, systolic BP > 160 mmHg or currently on antihypertensive therapy and evidence of end organ damage. Could be included if aged 55 to 59 years and meeting two or more criteria for end organ damage. patients Amlodipine plus benzapril (N = 5,744) VS Hydrochlorothiazide plus benzapril (n = 5,762) PRIMARY OUTCOME 9.6 Time to the composite of a CV event and death from CV cause HR 0.80, 95% CI, 0.72-0.90, P<0.001 11.8 SECONDARY OUTCOME 5.0 Death from CV causes plus nonfatal MI and nonfatal stroke % HR 0.79; P=0.002 6.3 8.6 Adverse cardiovascular events HR 0.83; P=0.002 10.3 Conclusion: The benazepril-amlodipine combination was superior to the benazepril-hydrochlorothiazide combination in reducing cardiovascular events in patients with hypertension who were at high risk for such events. Jamerson K, et al. NEJM, 2008; 359:2417-2428