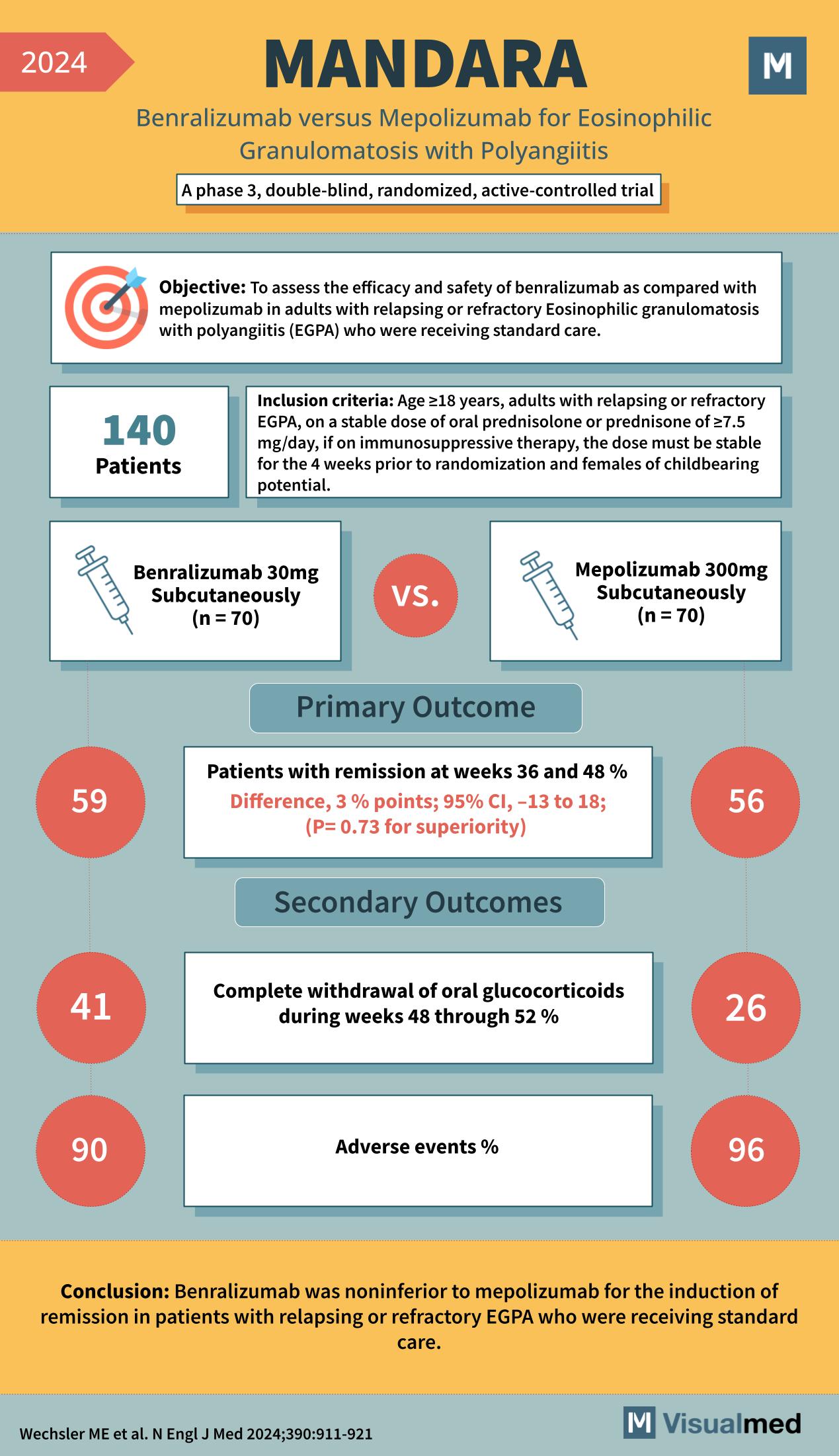
“MANDARA” study, a phase 3, double-blind, randomized, active-controlled trial, comparing Benralizumab versus Mepolizumab for Eosinophilic Granulomatosis with Polyangiitis (EGPA).
Objective: To assess the efficacy and safety of benralizumab as compared with mepolizumab in adults with relapsing or refractory EGPA who were receiving standard care.
Inclusion criteria: Age ≥18 years, adults with relapsing or refractory EGPA, on a stable dose of oral prednisolone or prednisone of ≥7.5 mg/day, if on immunosuppressive therapy, the dose must be stable for the 4 weeks prior to randomization, and females of childbearing potential.
Patients: 140 patients participated.
Treatment groups:
- Benralizumab 30mg Subcutaneously (n=70)
- Mepolizumab 300mg Subcutaneously (n=70)
Primary Outcome:
- Patients with remission at weeks 36 and 48 %.
- Difference: 3% points; 95% CI, -13 to 18; (P=0.73 for superiority)
Secondary Outcomes:
- Complete withdrawal of oral glucocorticoids during weeks 48 through 52 %.
- Adverse events %.
Results:
- 59% of patients in the Benralizumab group achieved remission.
- 56% of patients in the Mepolizumab group achieved remission.
- 41% in the Benralizumab group had complete withdrawal of oral glucocorticoids.
- 26% in the Mepolizumab group had complete withdrawal of oral glucocorticoids.
- 90% in the Benralizumab group experienced adverse events.
- 96% in the Mepolizumab group experienced adverse events.
Conclusion: Benralizumab was noninferior to mepolizumab for the induction of remission in patients with relapsing or refractory EGPA who were receiving standard care.
Wechsler ME et al. N Engl J Med 2024;390:911-921