WOSCOPS Trial Summary
“The WOSCOPS Trial: Pravastatin’s Role in Reducing the Incidence of Myocardial Infarction and Death from Cardiovascular Causes”
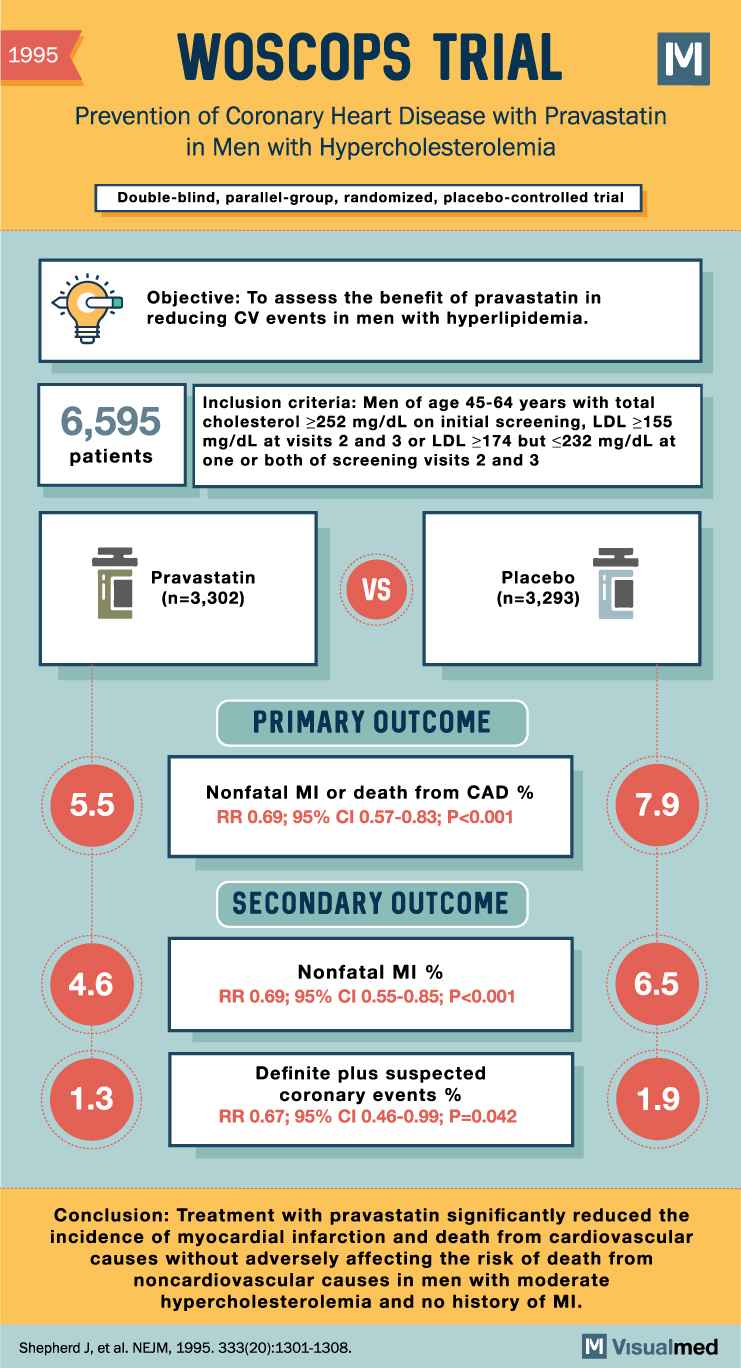
The West of Scotland Coronary Prevention Study (WOSCOPS) has significantly contributed to our understanding of the role of pravastatin in managing hypercholesterolemia and reducing the risk of coronary heart disease. This landmark double-blind study focused on men with hypercholesterolemia, aged 45 to 64, who had no history of myocardial infarction. The main goal was to ascertain whether the administration of pravastatin could reduce the combined incidence of nonfatal myocardial infarction and death from coronary heart disease.
In this trial, 6595 participants were randomly assigned to receive either pravastatin (40 mg each evening) or a placebo. The study had an average follow-up period of 4.9 years. Clinical end points were determined using medical records, electrocardiographic recordings, and the national death registry.
Results from the WOSCOPS trial were notable. Pravastatin effectively lowered plasma cholesterol levels by 20% and low-density lipoprotein cholesterol levels by 26%. In contrast, no significant changes were observed in the placebo group. The study reported 248 definite coronary events in the placebo group, compared to 174 in the pravastatin group. This indicated a relative reduction in risk with pravastatin of 31%, with a 95% confidence interval of 17% to 43%.
The WOSCOPS trial showed that pravastatin provided similar reductions in the risk of definite nonfatal myocardial infarctions and death from coronary heart disease. Specifically, a 31% reduction was reported for nonfatal myocardial infarctions, a 28% reduction for definite cases of death from coronary heart disease, and a 32% reduction for death from all cardiovascular causes. Importantly, there was no increase in deaths from noncardiovascular causes in the pravastatin group.
Overall, the results of the WOSCOPS trial demonstrated that treatment with pravastatin significantly reduced the incidence of myocardial infarction and death from cardiovascular causes without adversely affecting the risk of death from noncardiovascular causes. These findings highlighted the potential benefits of pravastatin for men with moderate hypercholesterolemia and no history of myocardial infarction, thus marking a significant advance in our understanding of primary prevention strategies for cardiovascular disease.