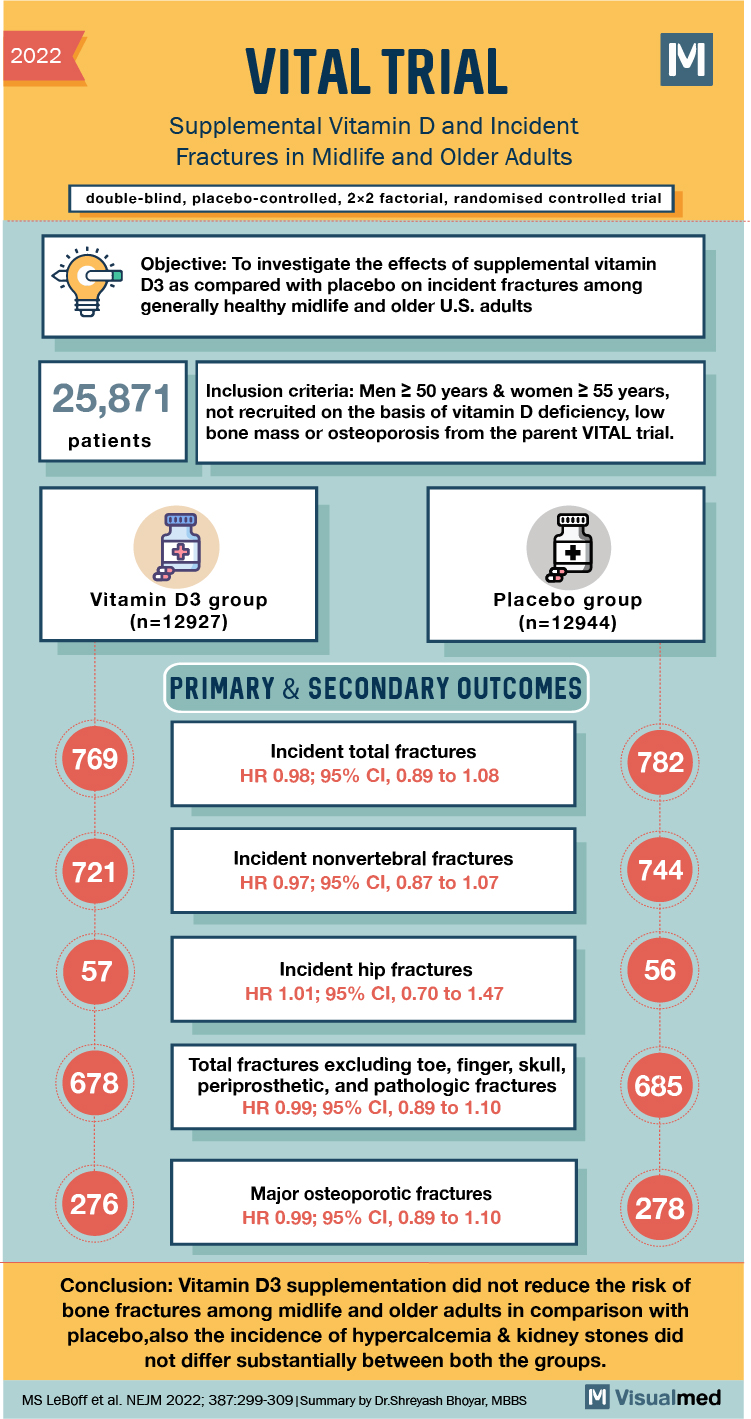
2022 VITAL TRIAL Supplemental Vitamin D and Incident Fractures in Midlife and Older Adults double-blind, placebo-controlled, 2×2 factorial, randomized controlled trial Objective: To investigate the effects of supplemental vitamin D3 as compared with placebo on incident fractures among generally healthy midlife and older U.S. adults 25,871 Inclusion criteria: Men 250 years & women 2 55 years, not recruited on the basis of vitamin D deficiency, low bone mass or osteoporosis from the parent VITAL trial. patients Vitamin D3 group (n=12927) Placebo group (n=12944) PRIMARY & SECONDARY OUTCOMES 769 Incident total fractures HR 0.98; 95% CI, 0.89 to 1.08 782 721 Incident nonvertebral fractures HR 0.97; 95% CI, 0.87 to 1.07 744 56 Incident hip fractures HR 1.01; 95% CI, 0.70 to 1.47 678 Total fractures excluding toe, finger, skull, periprosthetic, and pathologic fractures HR 0.99; 95% CI, 0.89 to 1.10 685 276 Major osteoporotic fractures HR 0.99; 95% CI, 0.89 to 1.10 278 Conclusion: Vitamin D3 supplementation did not reduce the risk of bone fractures among midlife and older adults in comparison with placebo,also the incidence of hypercalcemia & kidney stones did not differ substantially between both the groups. MS LeBoff et al. NEJM 2022; 387:299-309