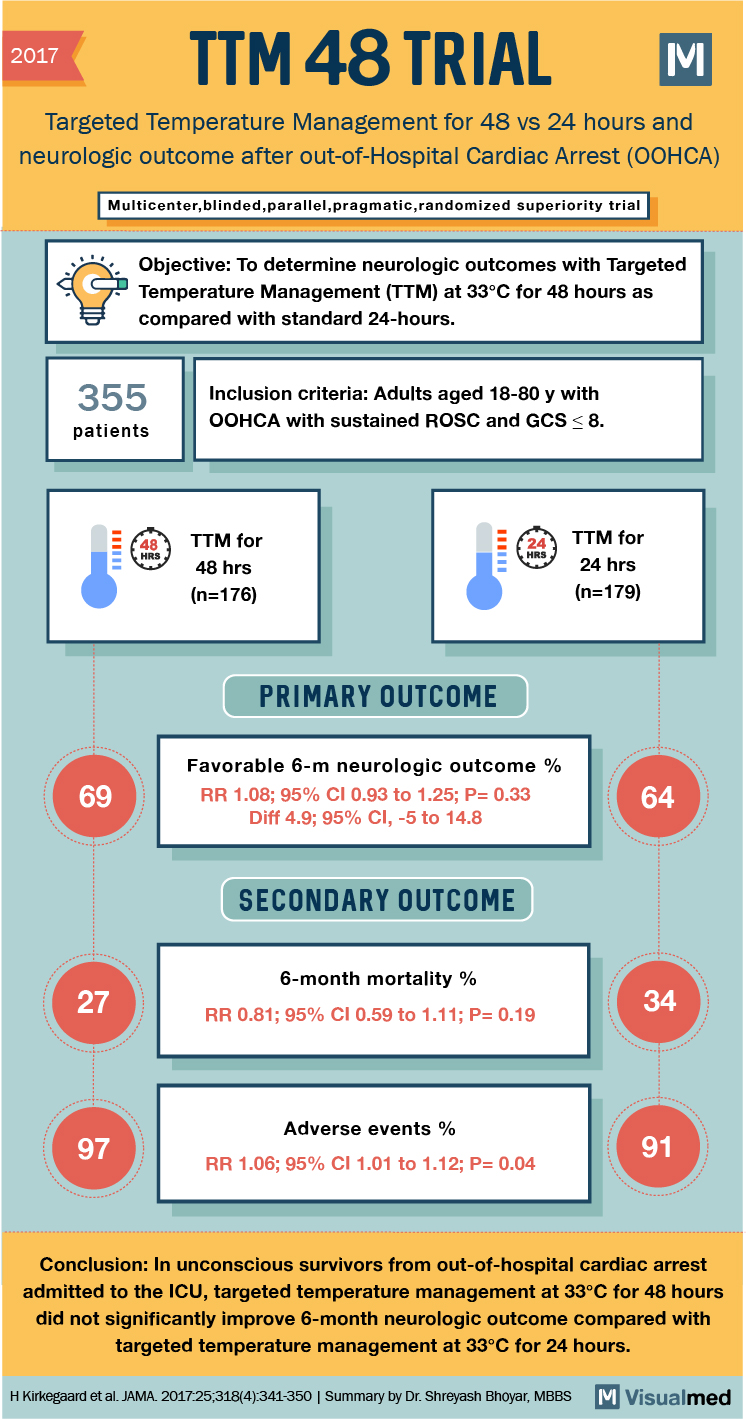
2017 2017 TTM 48 TRIAL M Targeted Temperature Management for 48 vs 24 hours and neurologic outcome after out-of-Hospital Cardiac Arrest (OOHCA) Multicenter,blinded,parallel,pragmatic,randomized superiority trial Objective: To determine neurologic outcomes with Targeted Temperature Management (TTM) at 33°C for 48 hours as compared with standard 24-hours. 355 Inclusion criteria: Adults aged 18-80 y with OOHCA with sustained ROSC and GCS < 8. booked a more con le set andere n patients 648 TTM for 48 hrs (n=176) TTM for 24 hrs (n=179) PRIMARY OUTCOME 69 Favorable 6-m neurologic outcome % RR 1.08; 95% CI 0.93 to 1.25; P= 0.33 Diff 4.9; 95% CI, -5 to 14.8 SECONDARY OUTCOME 6-month mortality % RR 0.81; 95% CI 0.59 to 1.11; P=0.19 Adverse events % RR 1.06; 95% CI 1.01 to 1.12; P=0.04 Conclusion: In unconscious survivors from out-of-hospital cardiac arrest admitted to the ICU, targeted temperature management at 33°C for 48 hours did not significantly improve 6-month neurologic outcome compared with targeted temperature management at 33°C for 24 hours. H Kirkegaard et al. JAMA. 2017:25,318(4):341-350