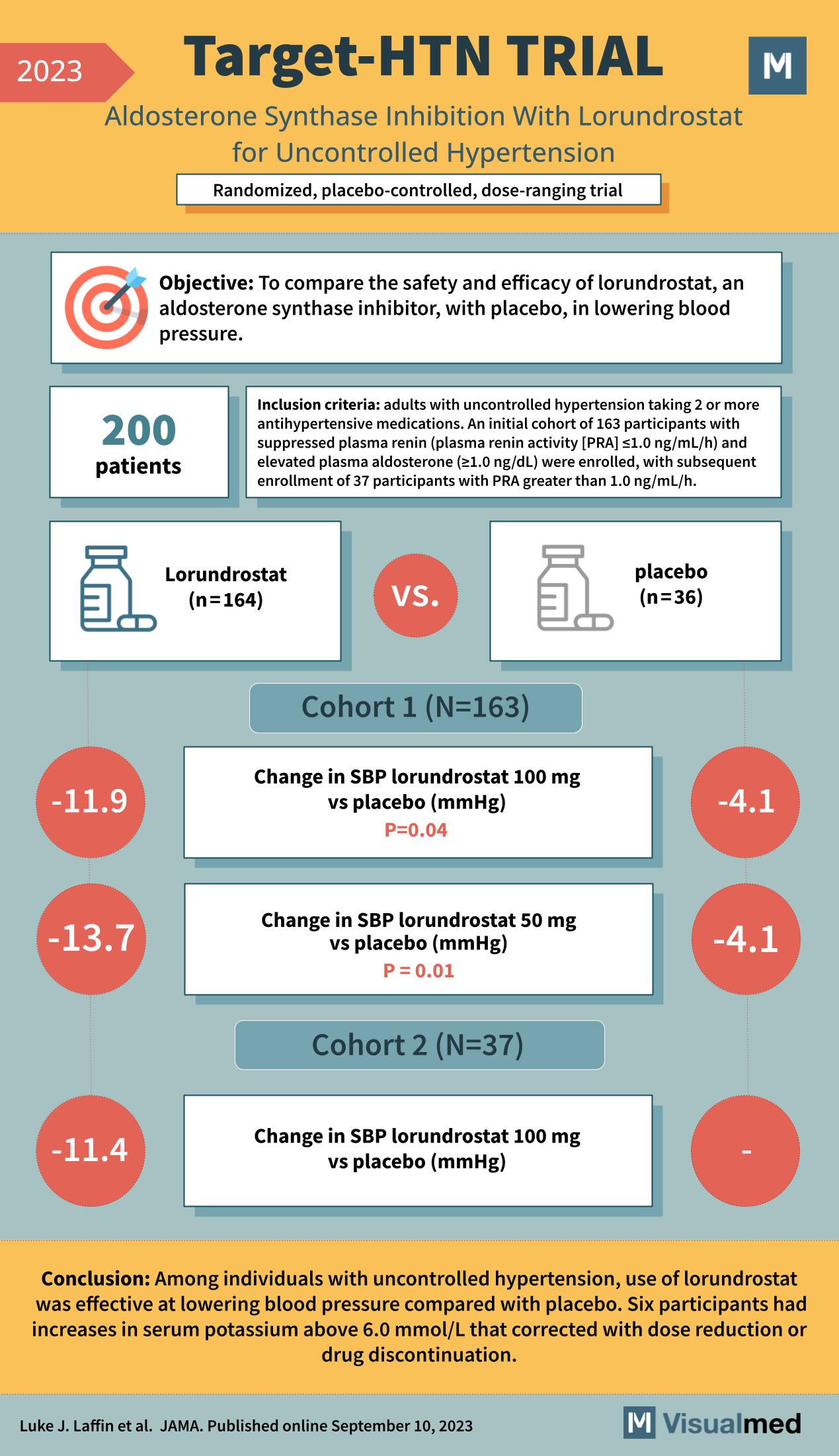
The TARGET-HTN trial, published in JAMA in September 2023, investigated the safety and efficacy of lorundrostat, an aldosterone synthase inhibitor, in treating patients with uncontrolled hypertension. This randomized, placebo-controlled, dose-ranging trial included 200 patients who were adults with uncontrolled hypertension taking two or more antihypertensive medications.
The study had two cohorts: the first cohort comprised 163 participants with suppressed plasma renin activity (PRA <1.0 ng/mL/h) and elevated plasma aldosterone (≥1.0 ng/dL), while the second cohort had 37 participants with PRA greater than 1.0 ng/mL/h. The participants were randomized to receive either lorundrostat or a placebo.
For Cohort 1, the change in systolic blood pressure (SBP) from baseline was significantly greater in the lorundrostat group compared to the placebo group, with a reduction of 11.9 mmHg for the 100 mg dose (p=0.04) and 13.7 mmHg for the 50 mg dose (p=0.01) of lorundrostat. Cohort 2, which had fewer participants, showed a reduction in SBP of 11.4 mmHg for the 100 mg dose of lorundrostat compared to placebo, although specific p-values for this cohort were not provided in the graphic.
The conclusion of the TARGET-HTN trial was that among individuals with uncontrolled hypertension, lorundrostat was effective at lowering blood pressure compared with placebo. It is important to note that six participants experienced increases in serum potassium above 6.0 mmol/L that required dose reduction or drug discontinuation.
This trial contributes valuable insights into the management of uncontrolled hypertension, suggesting that lorundrostat could be a promising therapeutic option. However, the potential for hyperkalemia necessitates careful monitoring and may limit the drug’s use in certain patient populations. The results of the TARGET-HTN trial support further investigation into the role of aldosterone inhibition in blood pressure management.