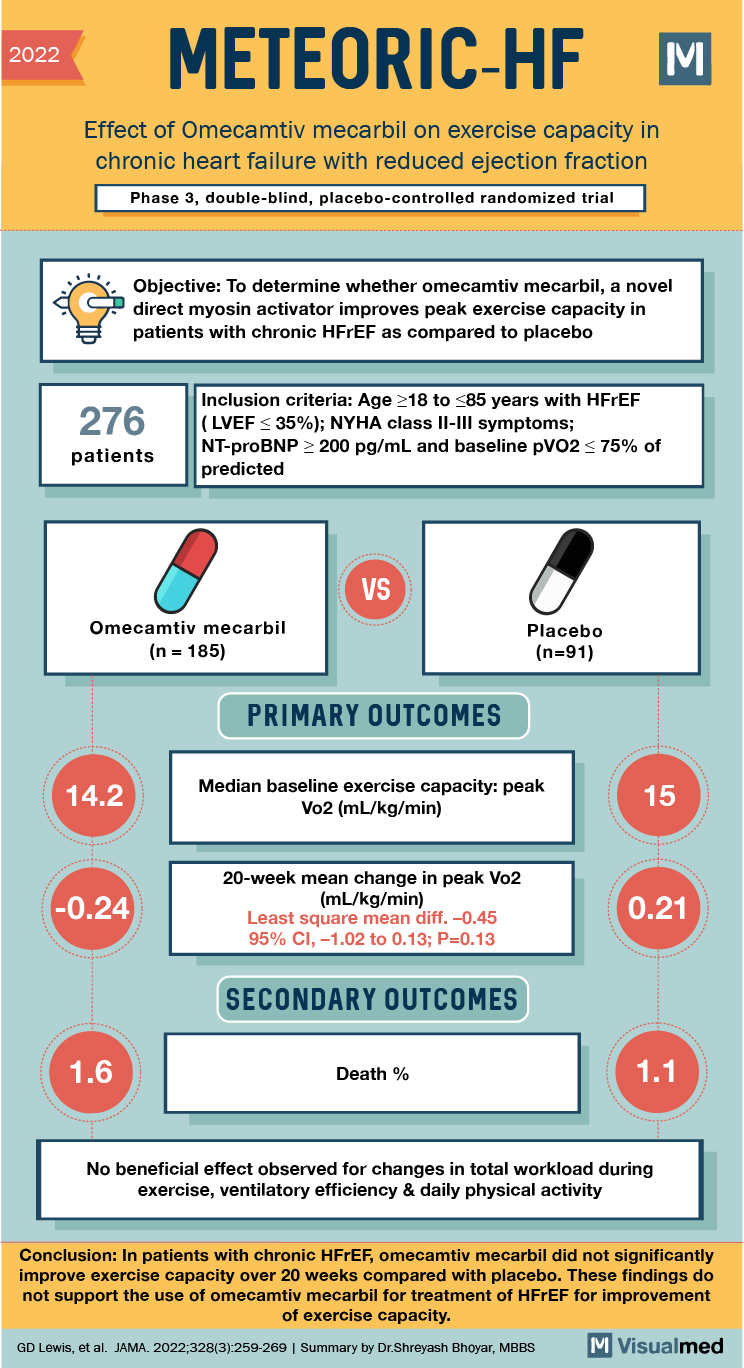
2022 METEORIC-HF Effect of Omecamtiv mecarbil on exercise capacity in chronic heart failure with reduced ejection fraction Phase 3, double-blind, placebo-controlled randomized trial a Objective: To determine whether omecamtiv mecarbil, a novel direct myosin activator improves peak exercise capacity in patients with chronic HFrEF as compared to placebo 276 Inclusion criteria: Age >18 to 385 years with HFrEF (LVEF <35%); NYHA class II-III symptoms; NT-proBNP > 200 pg/mL and baseline pVO2 < 75% of predicted patients VS Omecamtiv mecarbil (n = 185) Placebo (n=91) (PRIMARY OUTCOMES 14.2 Median baseline exercise capacity: peak Vo2 (ml/kg/min) -0.24 20-week mean change in peak Vo2 (mL/kg/min) Least square mean diff. -0.45 95% CI, -1.02 to 0.13; P=0.13 0.21 SECONDARY OUTCOMES Death % No beneficial effect observed for changes in total workload during exercise, ventilatory efficiency & daily physical activity Conclusion: In patients with chronic HFrEF, omecamtiv mecarbil did not significantly improve exercise capacity over 20 weeks compared with placebo. These findings do not support the use of omecamtiv mecarbil for treatment of HFrEF for improvement of exercise capacity. GD Lewis, et al. JAMA. 2022;328(3):259-269