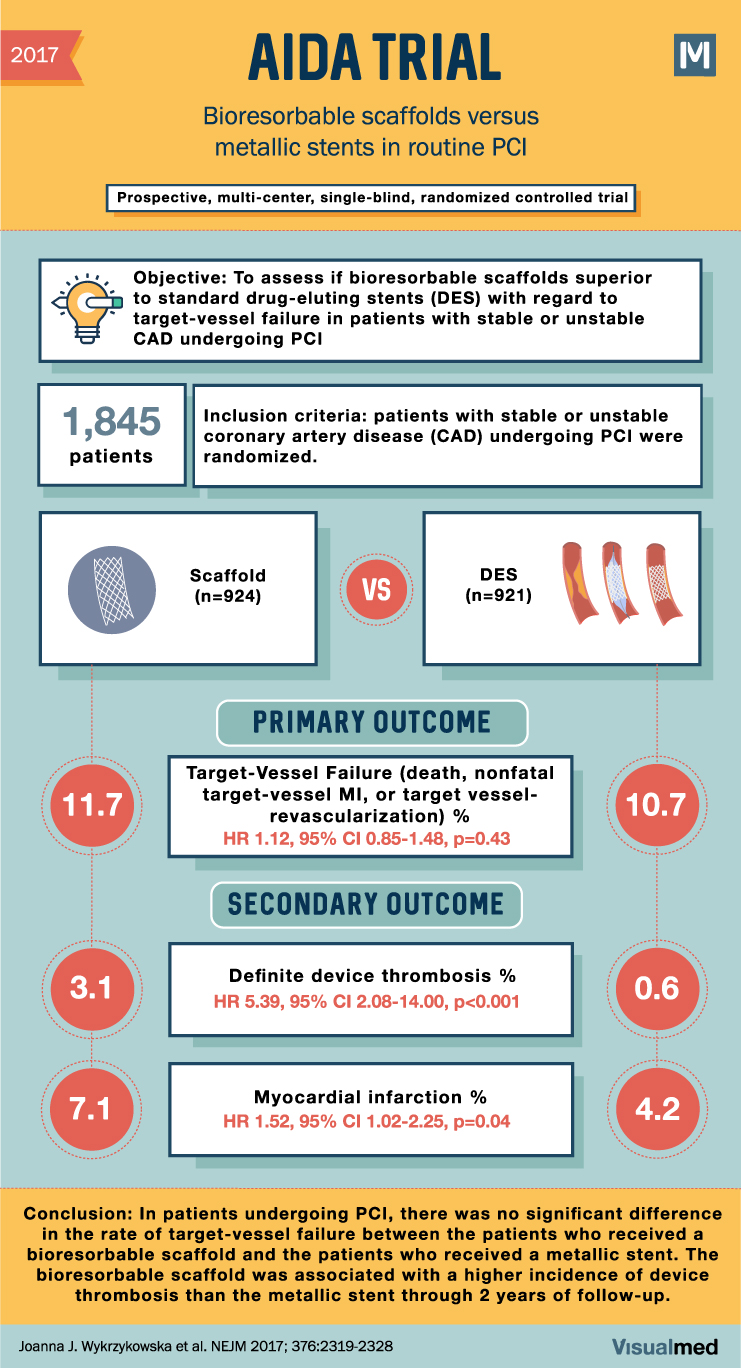
2017 AIDA TRIAL Bioresorbable scaffolds versus metallic stents in routine PCI Prospective, multi-center, single-blind, randomized controlled trial M Objective: To assess if bioresorbable scaffolds superior to standard drug-eluting stents (DES) with regard to target-vessel failure in patients with stable or unstable CAD undergoing PCI 1,845 Inclusion criteria: patients with stable or unstable patients coronary artery disease (CAD) undergoing PCI were randomized. 11.7 Scaffold (n=924) VS DES (n=921) PRIMARY OUTCOME Target-Vessel Failure (death, nonfatal target-vessel MI, or target vessel- revascularization) % HR 1.12, 95% CI 0.85-1.48, p=0.43 SECONDARY OUTCOME 10.7 3.1 Definite device thrombosis % HR 5.39, 95% CI 2.08-14.00, p<0.001 0.6 7.1 Myocardial infarction % 4.2 HR 1.52, 95% CI 1.02-2.25, p=0.04 Conclusion: In patients undergoing PCI, there was no significant difference in the rate of target-vessel failure between the patients who received a bioresorbable scaffold and the patients who received a metallic stent. The bioresorbable scaffold was associated with a higher incidence of device thrombosis than the metallic stent through 2 years of follow-up. Joanna J. Wykrzykowska et al. NEJM 2017; 376:2319-2328