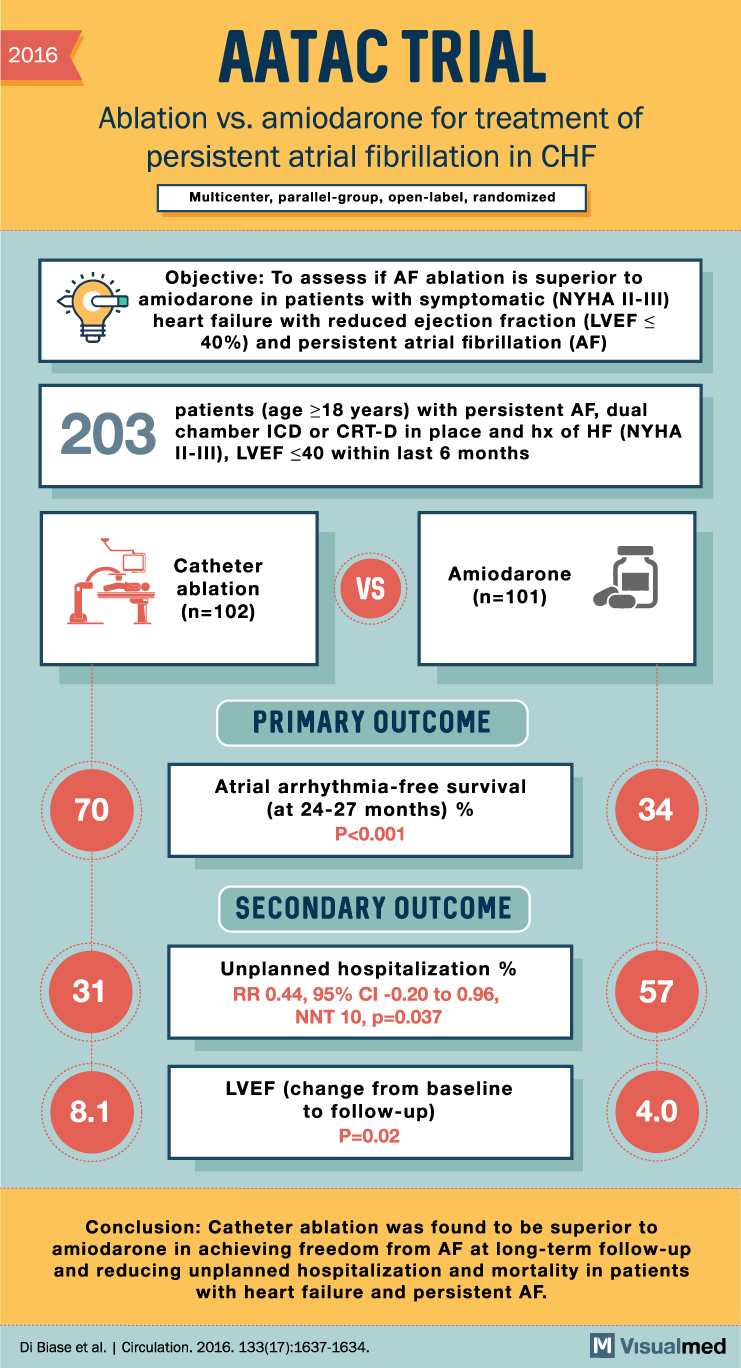
2016 AATAC TRIAL Ablation vs. amiodarone for treatment of persistent atrial fibrillation in CHF Multicenter, parallel-group, open-label, randomized Objective: To assess if AF ablation is superior to y amiodarone in patients with symptomatic (NYHA II-III) heart failure with reduced ejection fraction (LVEFS 40%) and persistent atrial fibrillation (AF) 203 patients (age 18 years) with persistent AF, dual chamber ICD or CRT-D in place and hx of HF (NYHA II-III), LVEF <40 within last 6 months Catheter ablation (n=102) VS Amiodarone (n=101) PRIMARY OUTCOME 70 Atrial arrhythmia-free survival (at 24-27 months) % P<0.001 SECONDARY OUTCOME 31 Unplanned hospitalization % RR 0.44, 95% CI -0.20 to 0.96, NNT 10, p=0.037 57 8.1 LVEF (change from baseline to follow-up) P=0.02 4.0 Conclusion: Catheter ablation was found to be superior to amiodarone in achieving freedom from AF at long-term follow-up and reducing unplanned hospitalization and mortality in patients with heart failure and persistent AF. Di Biase et al. Circulation. 2016. 133(17):1637-1634.